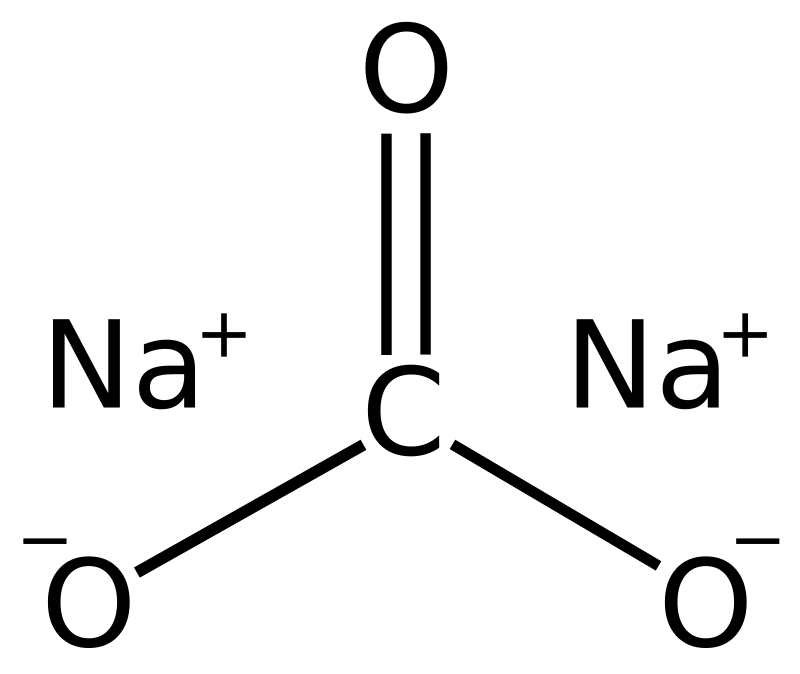
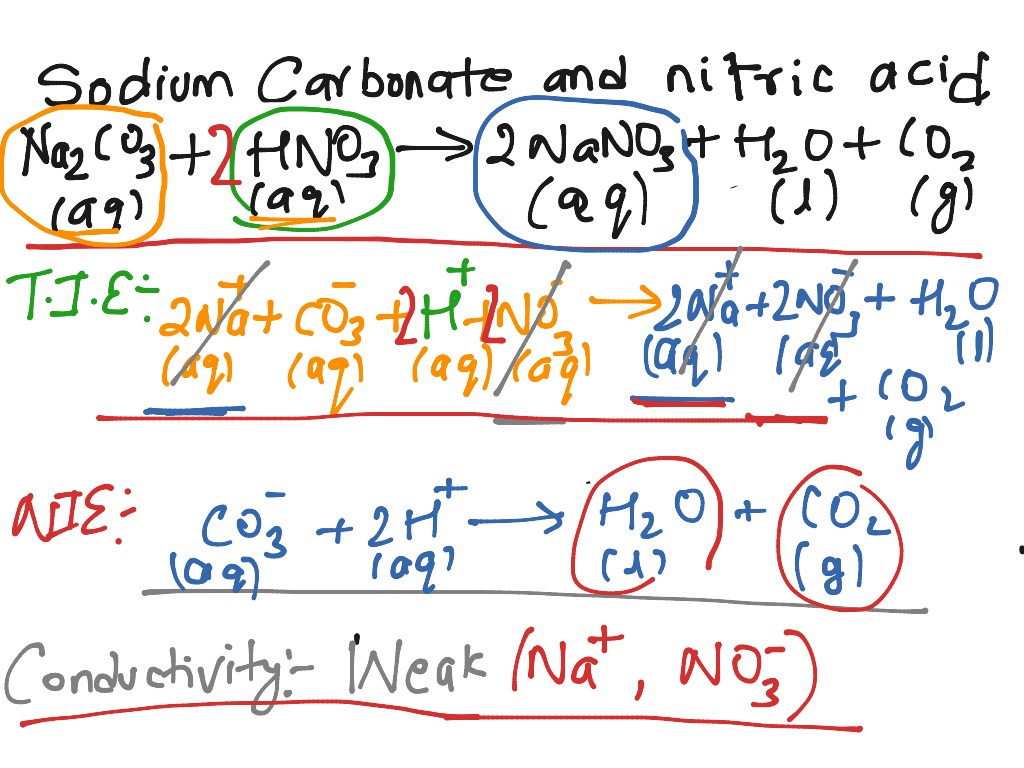
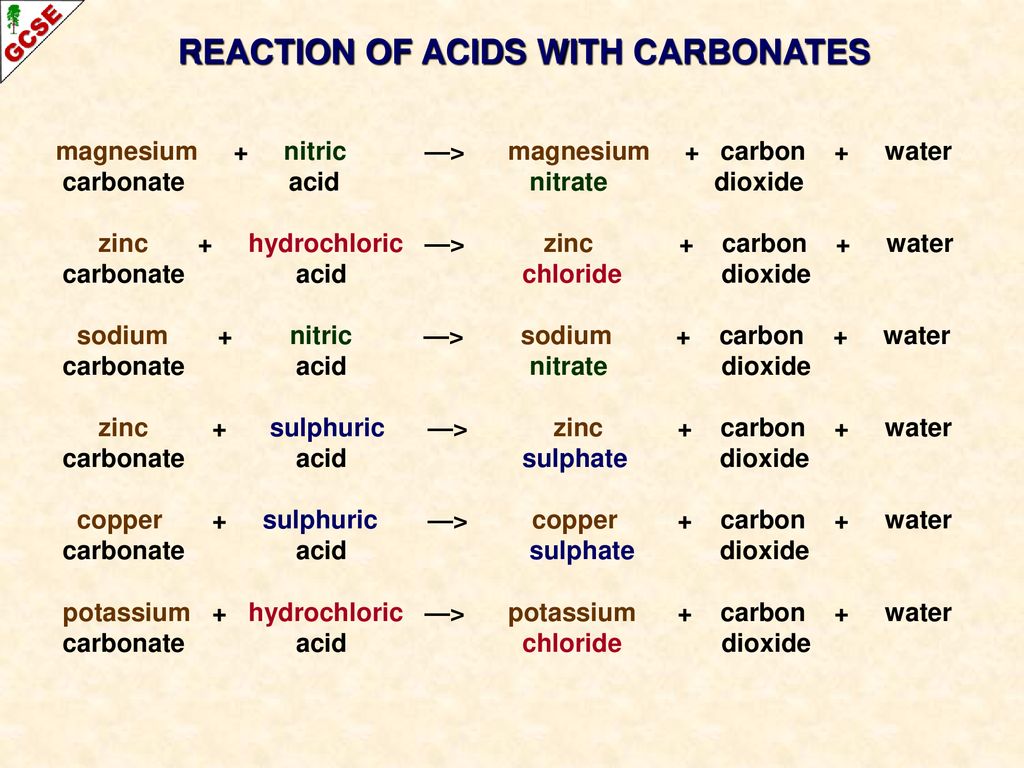
Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride

EP0858985A2 - Aqueous alkaline earth nitrate fertilizer composition and process for making same - Google Patents

Q4 Match the compounds in List I 1 to 20 with their correct formulas from in List II A to T List I 1...

Applied Sciences | Free Full-Text | Features of the Hydrosulfate Method for Processing Alumina-Containing Raw Materials in a Closed Reagent Cycle