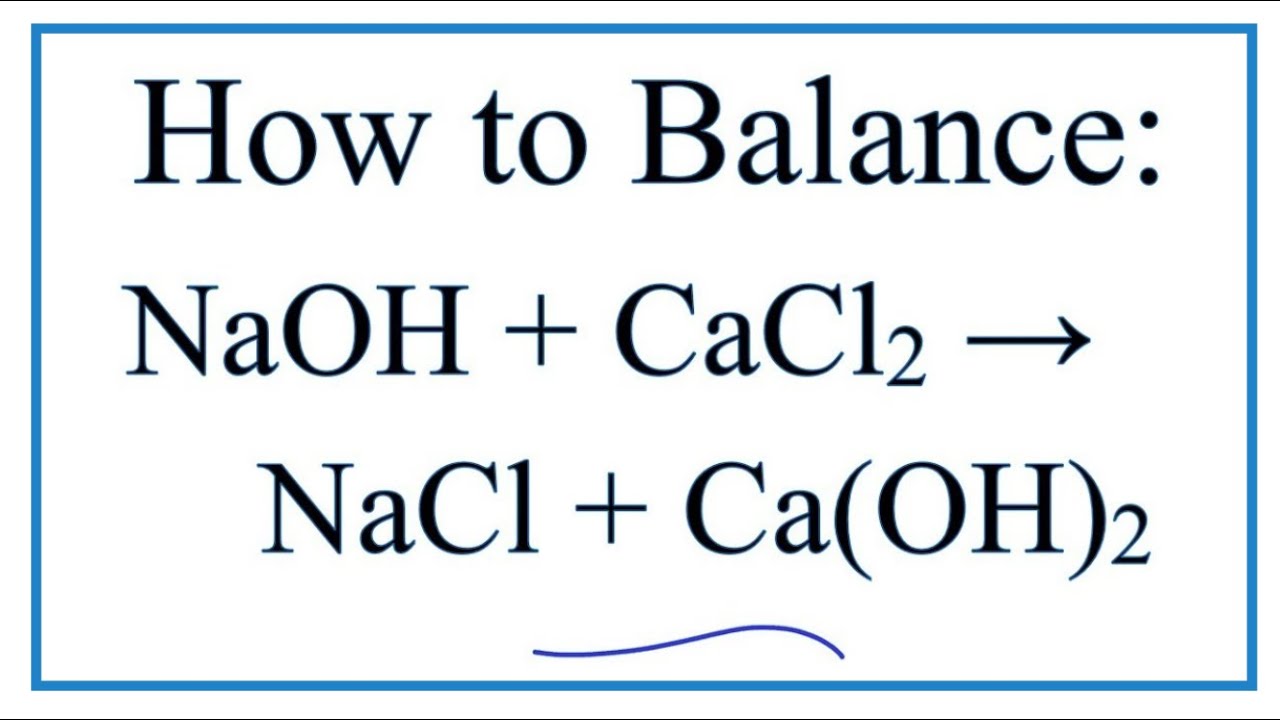
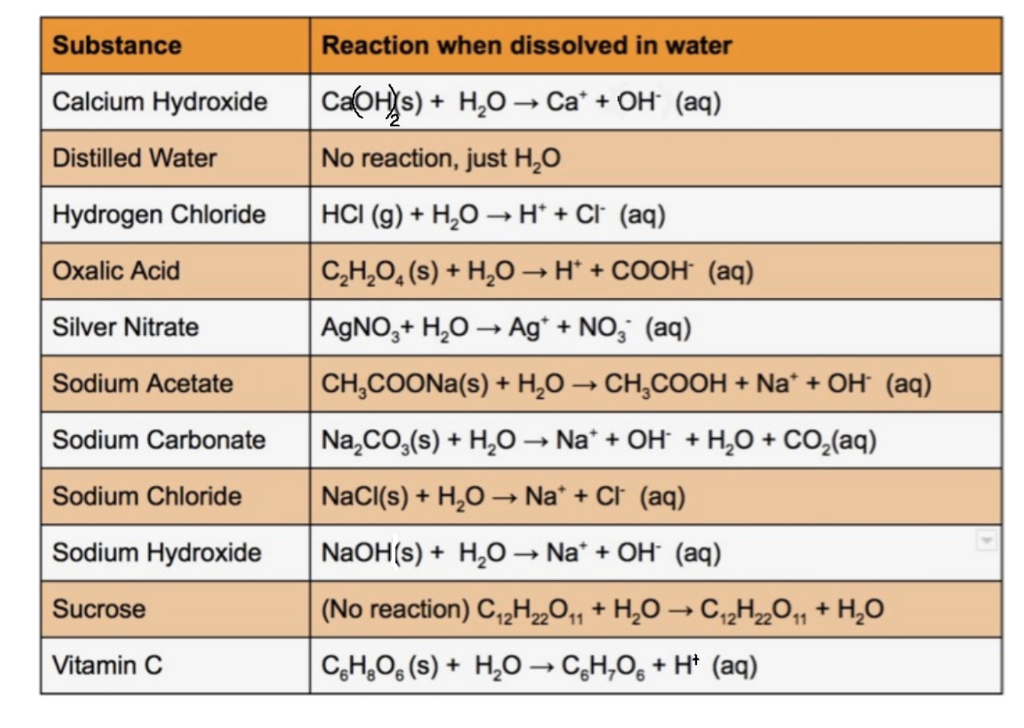
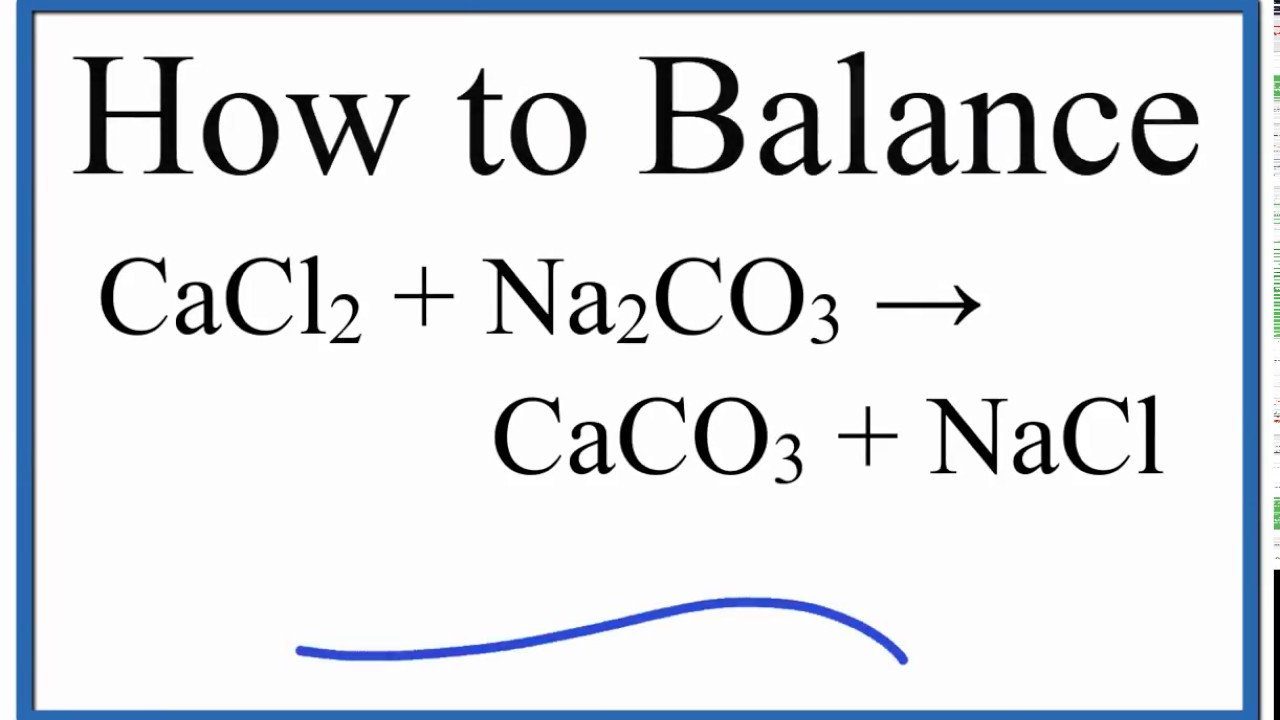Reaction of calcium carbonate minerals in sodium silicate solution and its role in alkali-activated systems - ScienceDirect
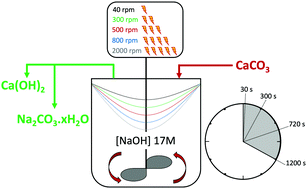
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing)

Question 53 Look at the figure and answer the following questions.a What change would you observe in the calcium Hydroxide solution taken in tube B?b Write the reaction involved in test tube
Calcium magnesium sodium carbonate hydrogen carbonate hydroxide (1:4:1:3:1:4) | C4H5CaMg4NaO16 | ChemSpider
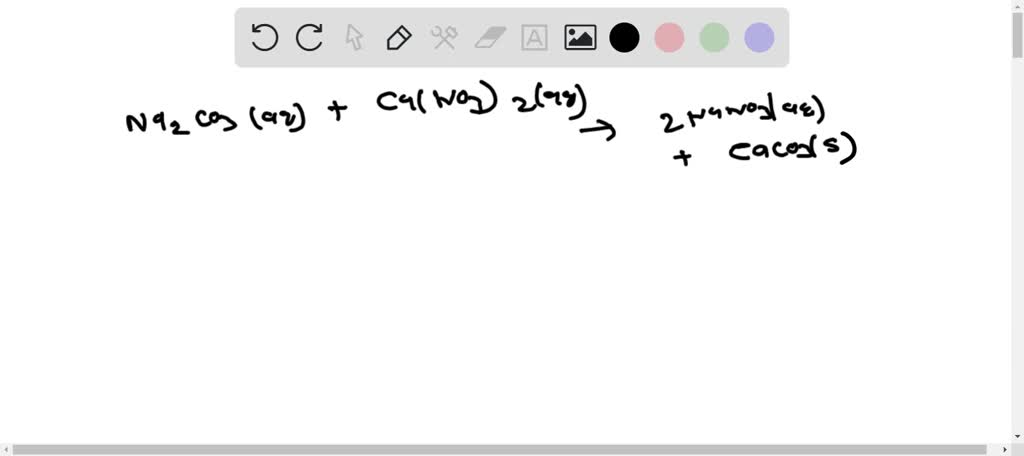
SOLVED: Write molecular, ionic and net ionic equations for following reactions Calcium Nitrate with Sodium Carbonate Calcium Nitrate with Sodium Hydroxide Calcium Nitrate with Trisodium Phosphate

SEM of CaCO 3 particles obtained by mixing calcium hydroxide solution... | Download Scientific Diagram
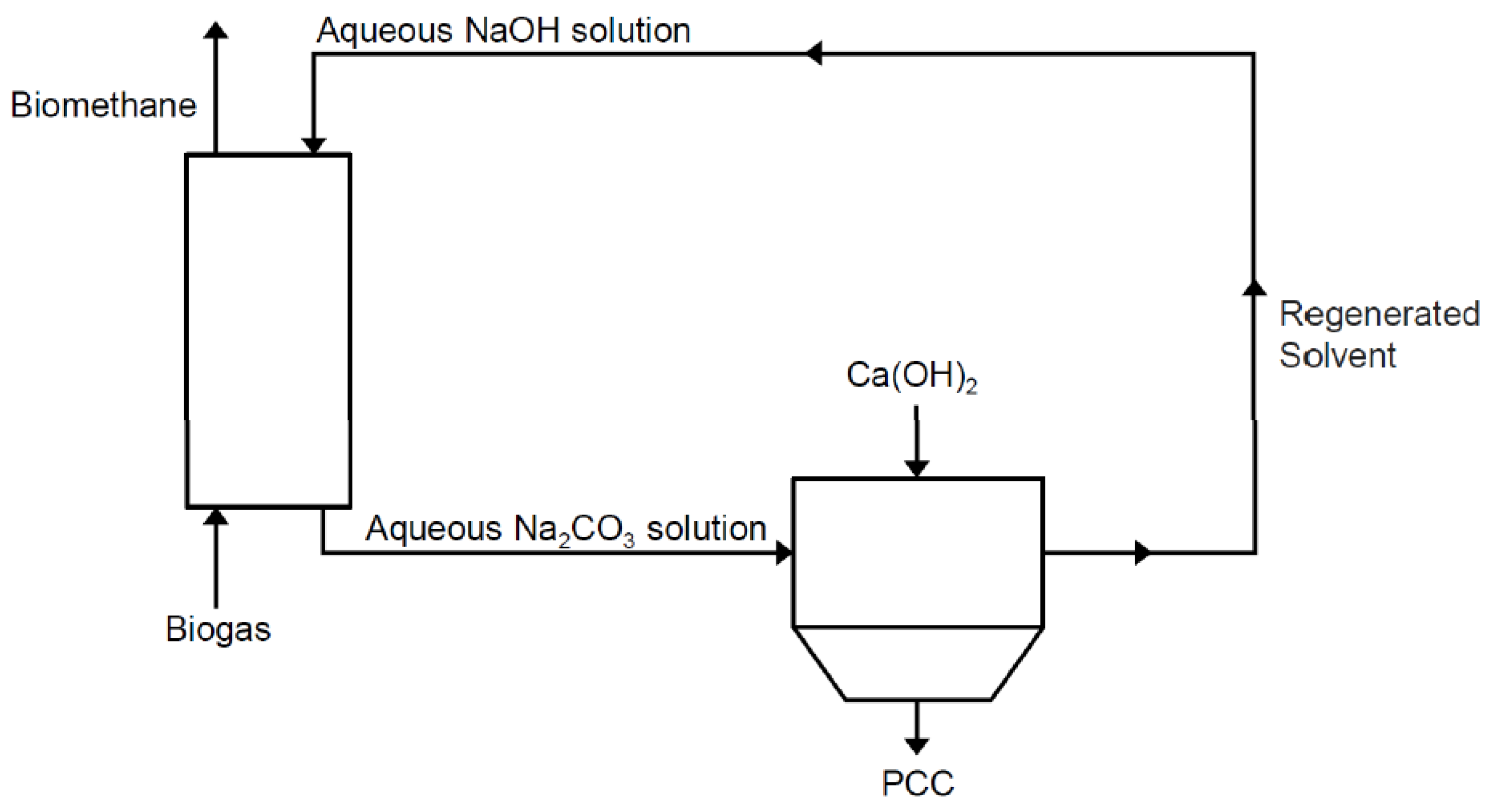
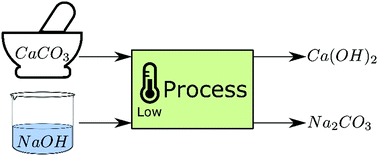
Processes | Free Full-Text | Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters

Look at below figure and answer the following questions What change would you observe in the calcium hydroxide solution taken in tube B? Write the reaction involved in test tubes A and

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride

Write formulae of the following compounds.Sodium phosphate, Aluminium chloride, Calcium oxide, Potassium nitrate, Ammonium hydroxide

SOLVED: If 3.5 moles of the sodium carbonate react with excess calcium carbonate, how many grams of sodium hydroxide will be produced? Na2co3 + Ca(OH)2 NaOH + Caco3