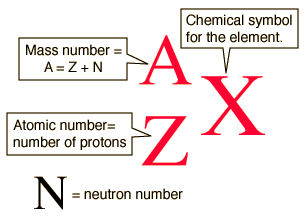Chapter 4 – Atomic Structure. Greek Atomic Theory (400 BC) “There is no smallest among the small and no largest among the large, but always something. - ppt download
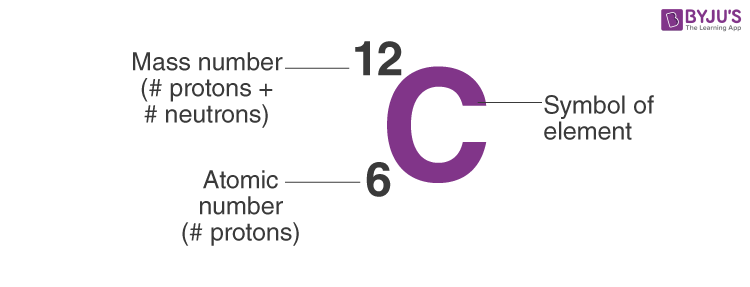
Carbon Protons - What are Protons, Number of protons in Carbon atom, and Uses of Carbon atom along with some FAQs

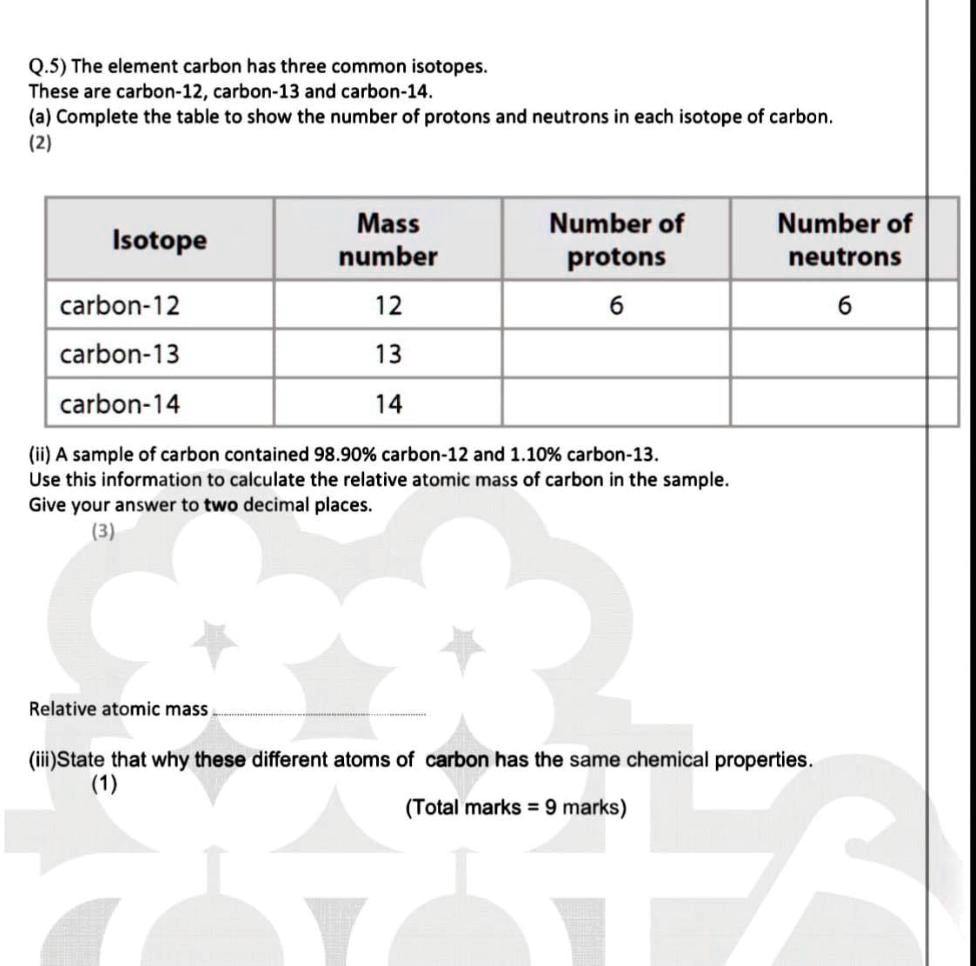
Question Video: Comparing the Atomic Number and Mass Number of Three Atoms Given the Number of Protons and Neutrons Each Contains | Nagwa

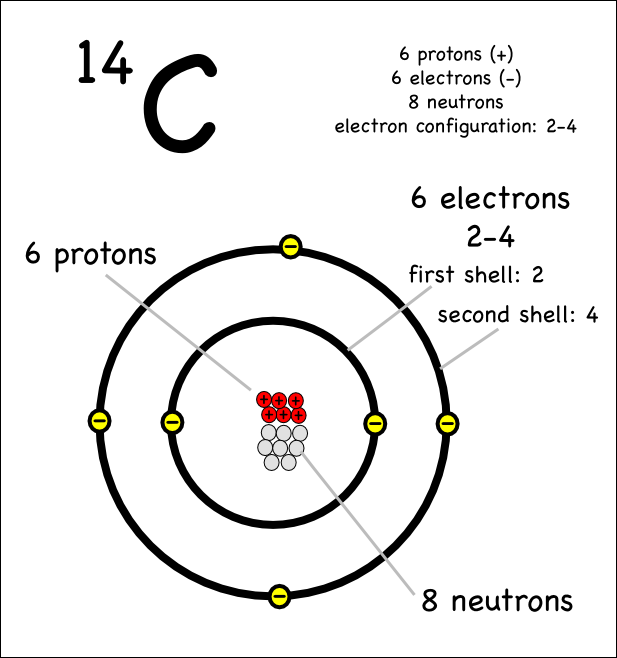
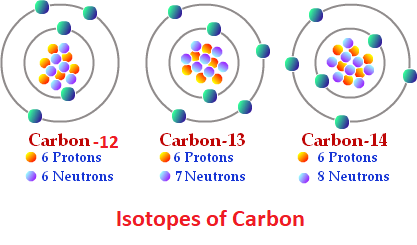
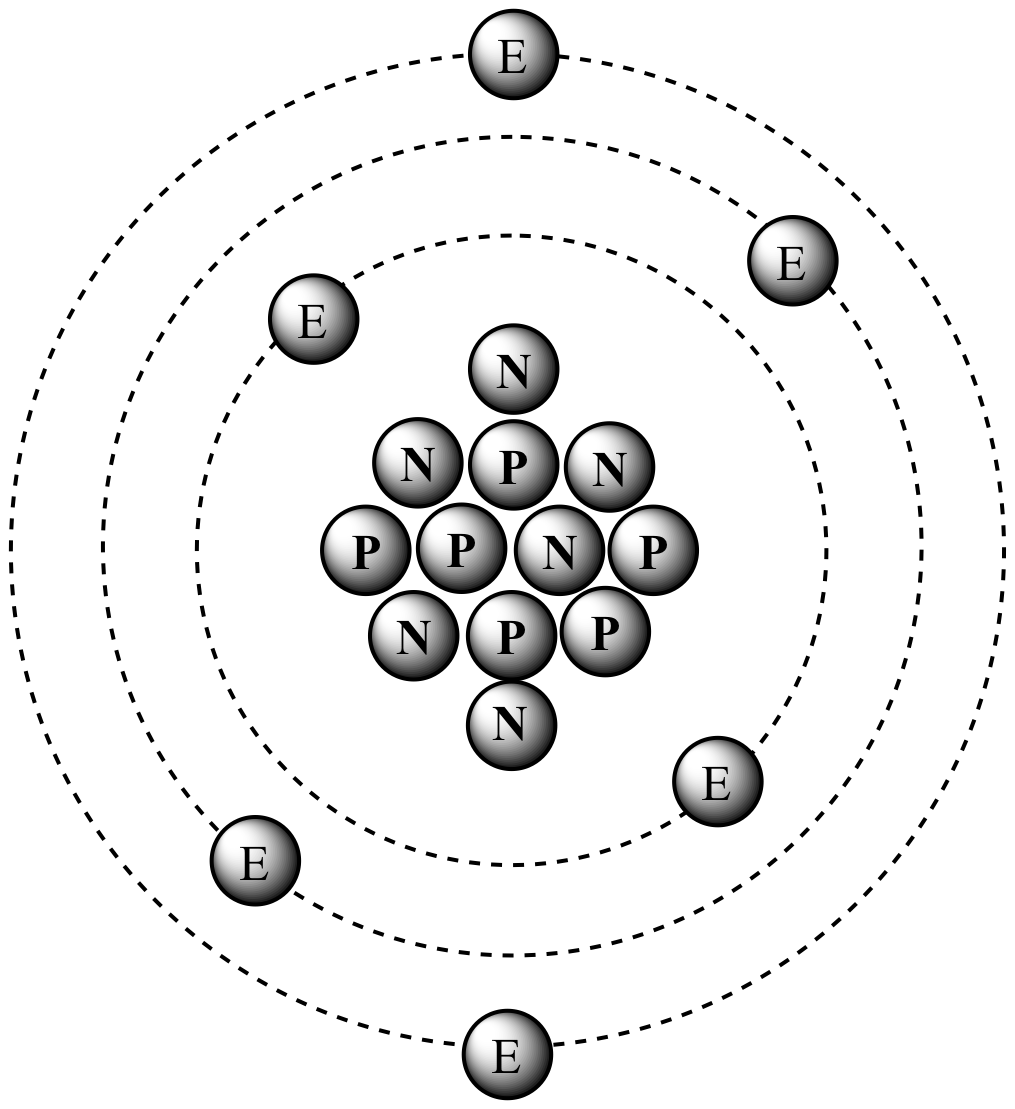
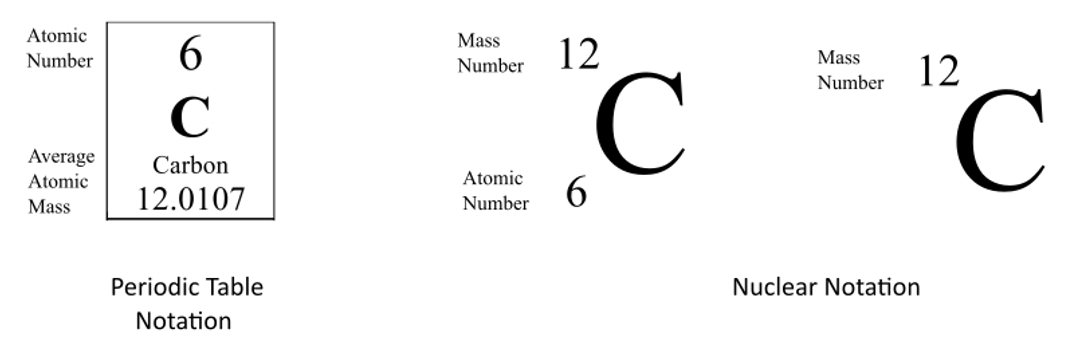
Atomic notation X A Z X = Symbol (C, Au) A = Atomic Mass Number = #nucleons (Protons + Neutrons) Z = Atomic Number = #protons C 12 6 Carbon A = 12, Z = - ppt download
![Chemistry Problems - ☆Carbon☆ Name: #Carbon Atomic Number: 6 Element Symbol: C Group: 14, Period: 2, Block: p Element Family: nonmetal Atomic Mass: [12.0096; 12.0116], use 12.011. #Electron_Configuration: [He]2s2 2p2 or 1s2 Chemistry Problems - ☆Carbon☆ Name: #Carbon Atomic Number: 6 Element Symbol: C Group: 14, Period: 2, Block: p Element Family: nonmetal Atomic Mass: [12.0096; 12.0116], use 12.011. #Electron_Configuration: [He]2s2 2p2 or 1s2](https://lookaside.fbsbx.com/lookaside/crawler/media/?media_id=115434776768821)
Chemistry Problems - ☆Carbon☆ Name: #Carbon Atomic Number: 6 Element Symbol: C Group: 14, Period: 2, Block: p Element Family: nonmetal Atomic Mass: [12.0096; 12.0116], use 12.011. #Electron_Configuration: [He]2s2 2p2 or 1s2
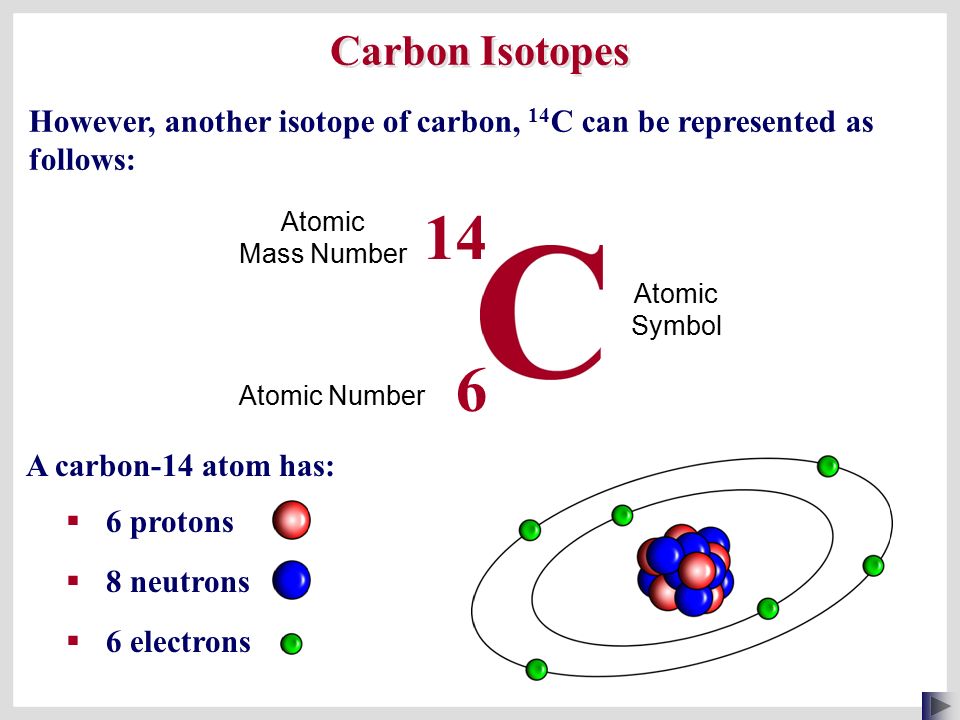
:max_bytes(150000):strip_icc()/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)