Write Chemical reaction of iron (fe),magnesium (mg) and calcium (ca) with hydrochloric acid (Hcl) and - Brainly.in
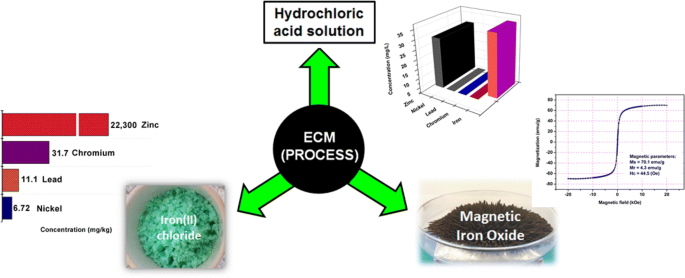
Reuse of Waste Pickling Acid for the Production of Hydrochloric Acid Solution, Iron(II) Chloride and Magnetic Iron Oxide: An Eco-Friendly Process | SpringerLink

SOLVED: Solid iron (Il) sulfide reacts with hydrochloric acid to form hydrogen sulfide gas and aqueous iron (II) chloride: FeS (s) + 2 HCI (aq) –> H2S (g) + FeCl2 (aq) If
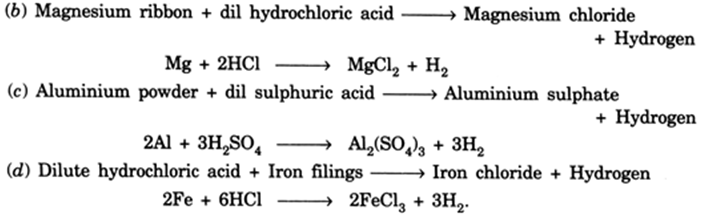
Write word equations and then balanced equations for the reaction taking place when:(a) dilute sulphuric acid reacts with zinc granules.(b) dilute hydrochloric acid reacts with magnesium ribbon.(c) dilute sulphuric acid reacts with

Effectiveness of Iron(II) Removal from Spent Hydrochloric Acid Using... | Download Scientific Diagram

Topic 9 ReactionsofAcids. Acids and Metals Think of the effect of acid rain on iron bridges and cars. When the acid rain falls on them a chemical reaction. - ppt download
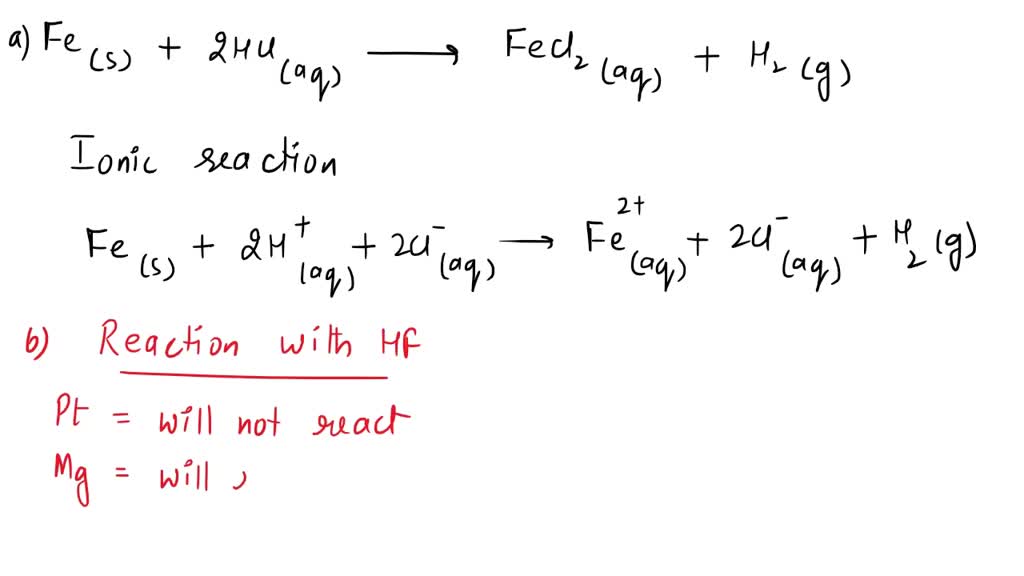
SOLVED: Write the net ionic equation for the reaction of iron with hydrochloric acid. Include phases. net ionic equation: Hydrofluoric acid reacts with metals in a similar fashion to hydrochloric acid. Predict

What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer.a)Iron salt and water are produced.b)Chlorine gas and iron hydroxide are produced.c)No reaction takes place.d)Hydrogen gas and iron
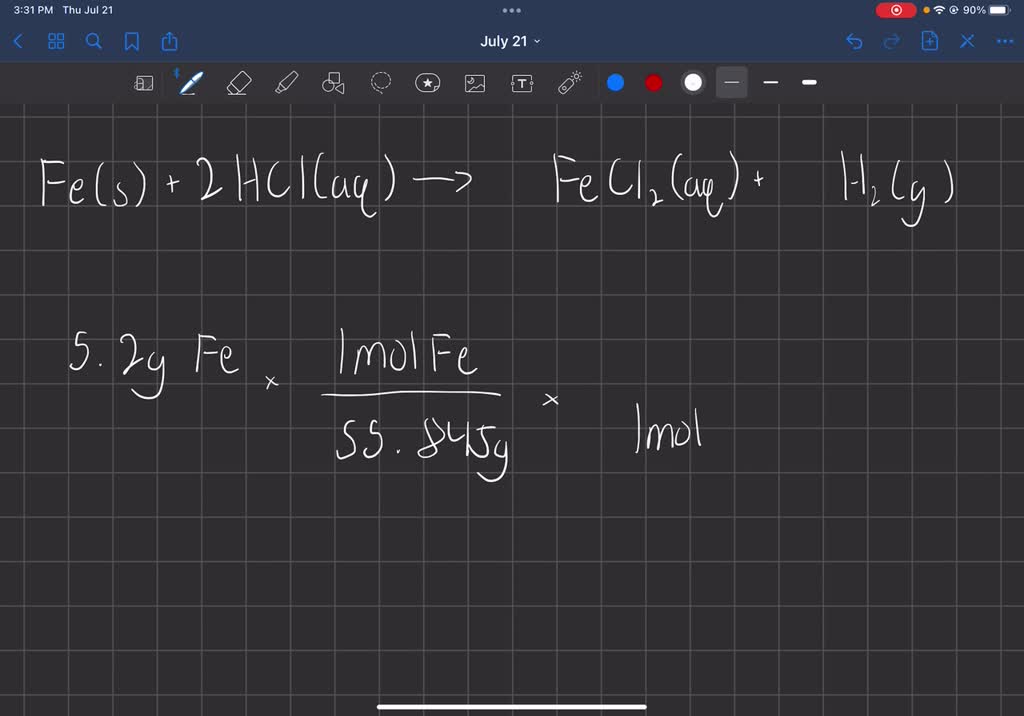
SOLVED: Iron reacts with hydrochloric acid to produce iron(II) chloride and hydrogen gas. Fe(s) + HCl(aq) → FeCl2(aq) + H2(g) What mass of H2(g) is produced from the reaction of 5.2 g

What happens when dilute hydrochloric acid is added to iron fillings? Tick the correct answer. (i) Hydrogen gas and iron chloride are produced. (ii) Chlorine gas and iron hydroxide are produced. (iii)

organic chemistry - Preference for tin or iron in the reduction of nitrobenzene - Chemistry Stack Exchange