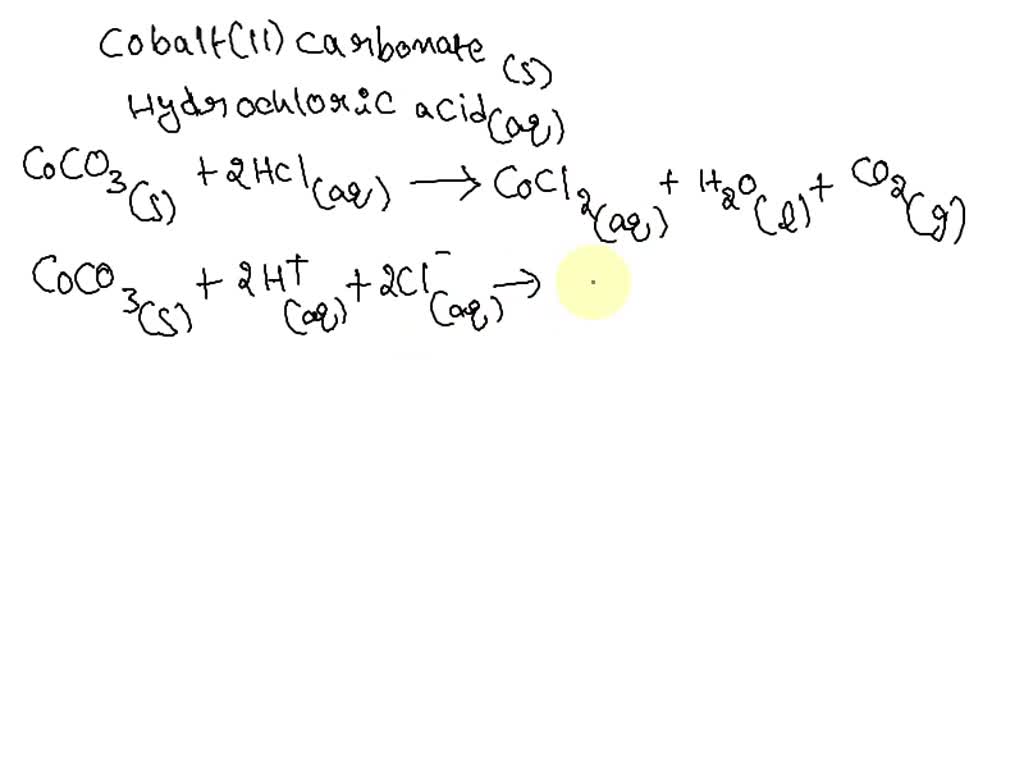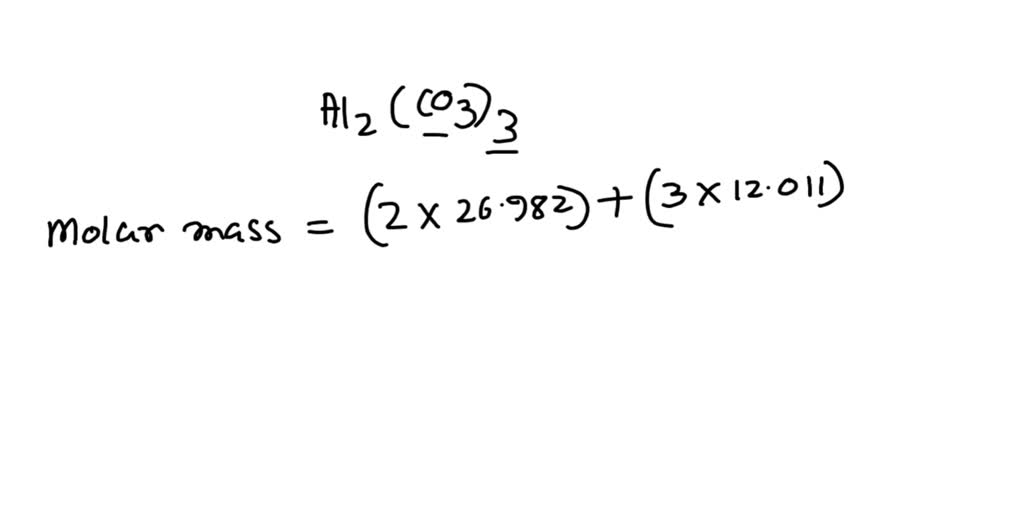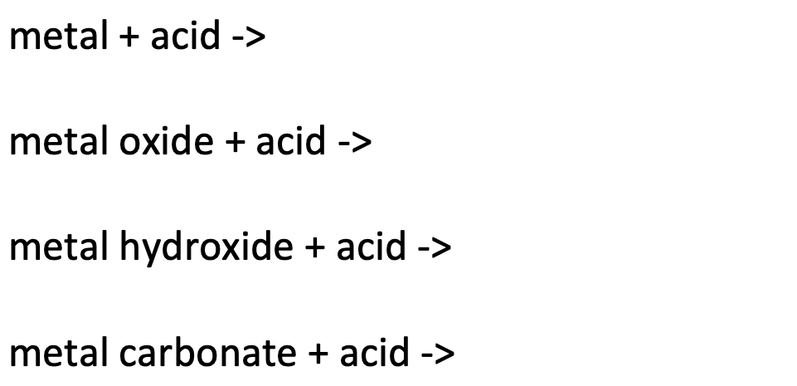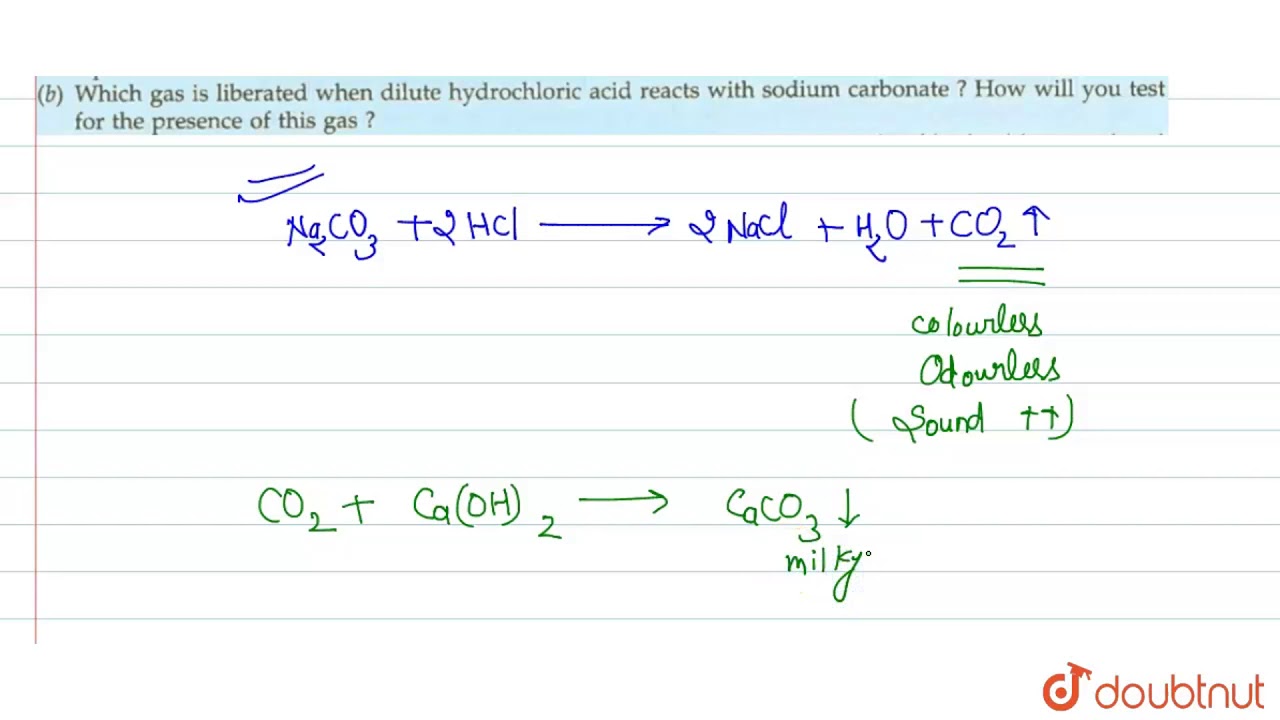
a). What happens when dilute hydrochloric acid is added to sodium carbonate? Write a balanced - YouTube

Model Experiment of Thermal Runaway Reactions Using the Aluminum–Hydrochloric Acid Reaction | Journal of Chemical Education

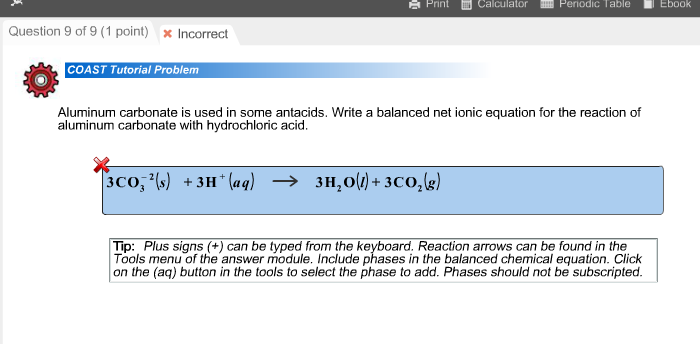
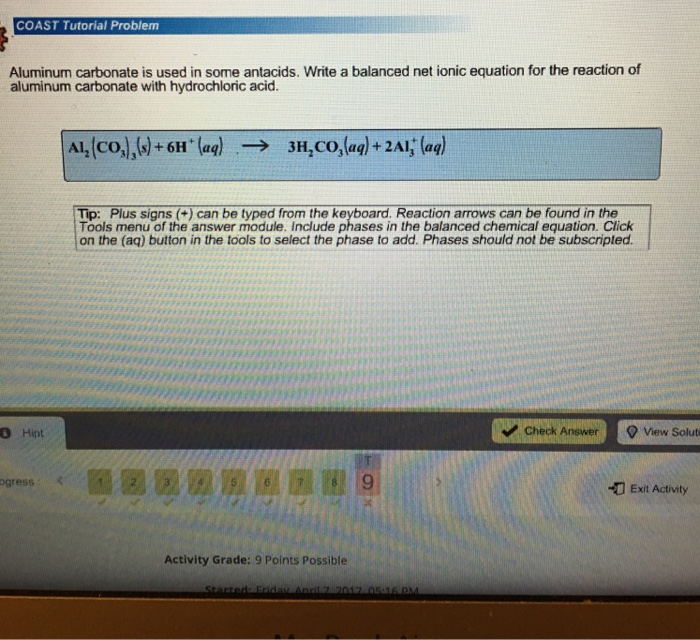
How to Balance Al2(CO3)3 + HCl = AlCl3 + H2O + CO2 (Aluminum carbonate + Hydrochloric acid) - YouTube

Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride

Write the balanced chemical equations for the following reactions.A Calcium hydroxide + Carbon dioxide → Calcium carbonate + waterB Zinc + Silver nitrate → Zinc nitrate + SilverC Aluminium + copper chloride
Write balanced chemical equations for the following reactions: i. Dilute sulphuric acid reacts with aluminium powder. - Sarthaks eConnect | Largest Online Education Community

How to Balance Al2(CO3)3 + HCl = AlCl3 + H2O + CO2 (Aluminum carbonate + Hydrochloric acid) - YouTube

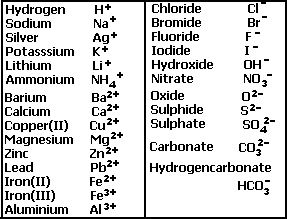
Reactions of hydrochloric sulfuric nitric acids with metals oxides hydroxides carbonates hydrogencarbonates word/symbol equations redox reaction half equations gcse chemistry revision notes igcse KS3 KS4 Science