Negative ion Formula in compoundcharge Oxide Hydroxide Nitrate NO 3 -1 Sulphate Carbonate. - ppt download

Naming and writing formulas. Ammonium NH 4 1+ Calcium Ca 2+ Iron (II) Fe 2+ Iron (III) Fe 3+ Sodium Na 1+ Tin (IV) Sn 4+ Acetate C 2 H 3 O 2 1- Carbonate. - ppt download

write the chemical formulae of the following compounds 1 ammonium sulphate 2 magnesium carbonate - Brainly.in
![If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16] If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]](https://dwes9vv9u0550.cloudfront.net/images/3796715/307077b2-1bb0-4bde-a9d0-fe5f6659fec9.jpg)
If the relative molecular mass of ammonium nitrate is 80, the percentage of nitrogen and oxygen in ammonium nitrate is: [N = 14, H = 1, O = 16]

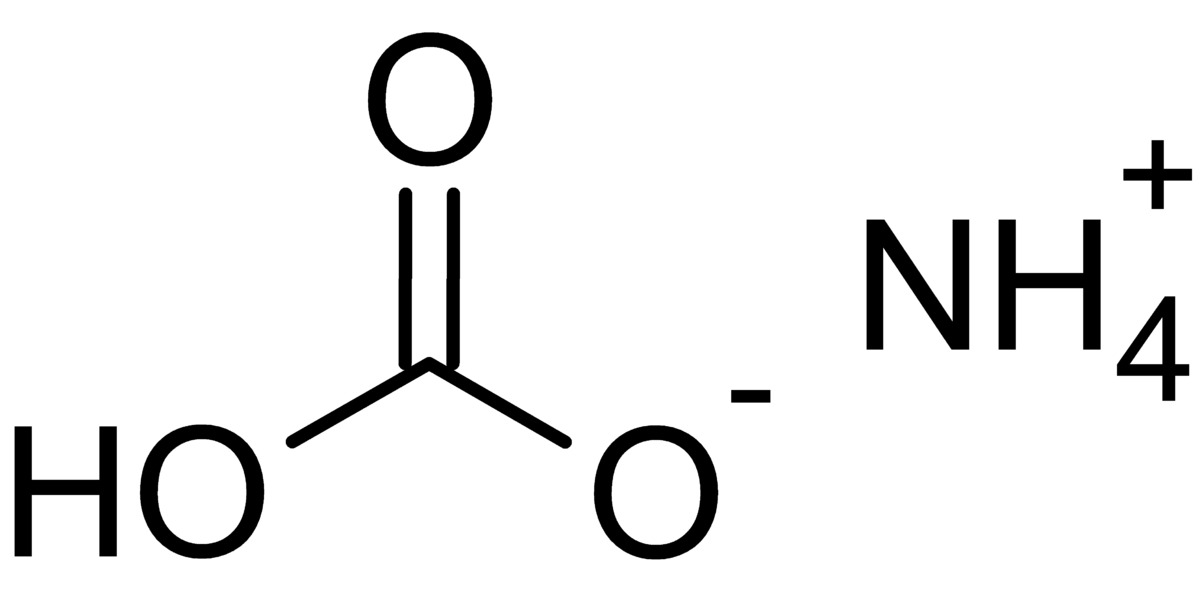
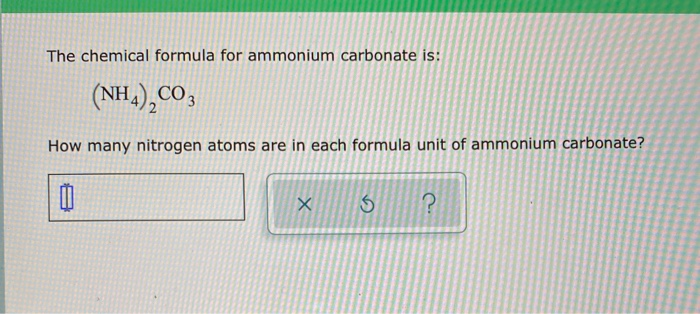
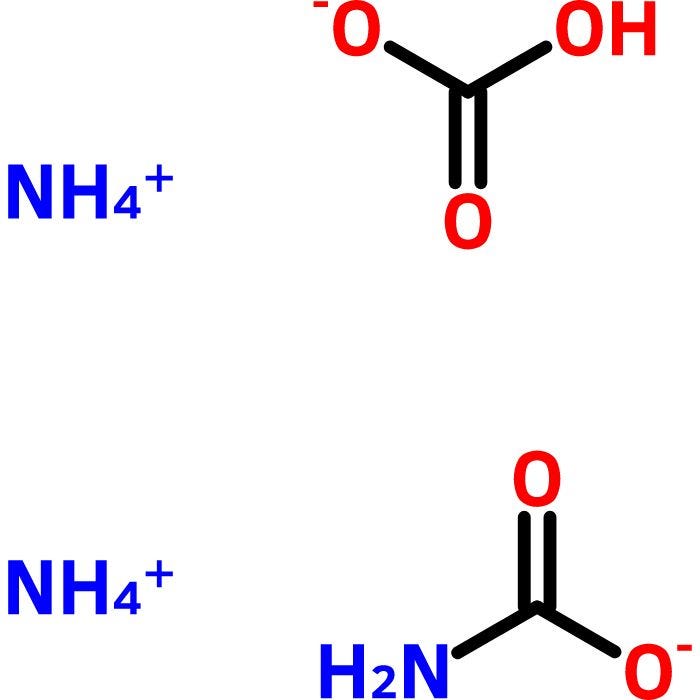
SOLVED: Ammonium carbonate, or (NH4 )2CO3 , a white solid that decomposes on warming, is a component of baking powder. a) How many formula units are in 41.6 g of (NH4 )2CO3 ?
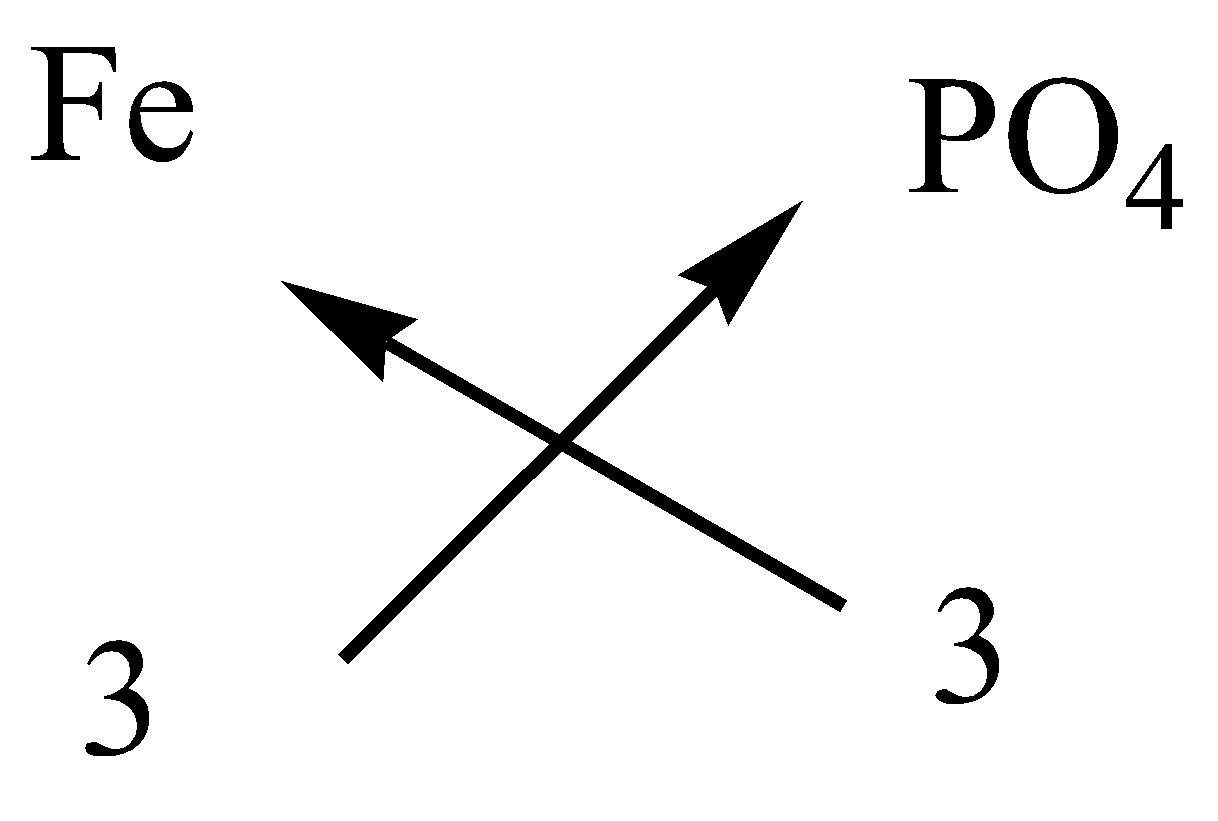
Write the formula of the following compounds by criss cross methodA.Ammonium carbonateB.Calcium bicarbonateC.Ferric phosphateD.Potassium sulfateE.Sodium zincate

Deduce the formula of: (a) Ammonium carbonate (b) Aluminium carbonate - Chemistry - Atoms and Molecules - 16513901 | Meritnation.com



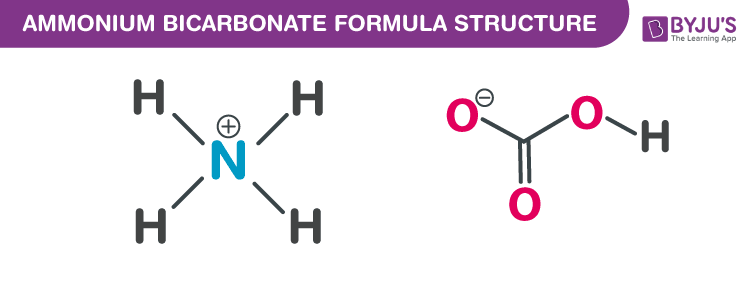

![Ammonium Bicarbonate | [NH4]HCO3 - PubChem Ammonium Bicarbonate | [NH4]HCO3 - PubChem](https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=14013&t=l)

![Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes Ammonium Carbonate [(NH4)2CO3] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2023/03/ammonium-carbonate-molecular-weight-calculation-300x198.jpg)