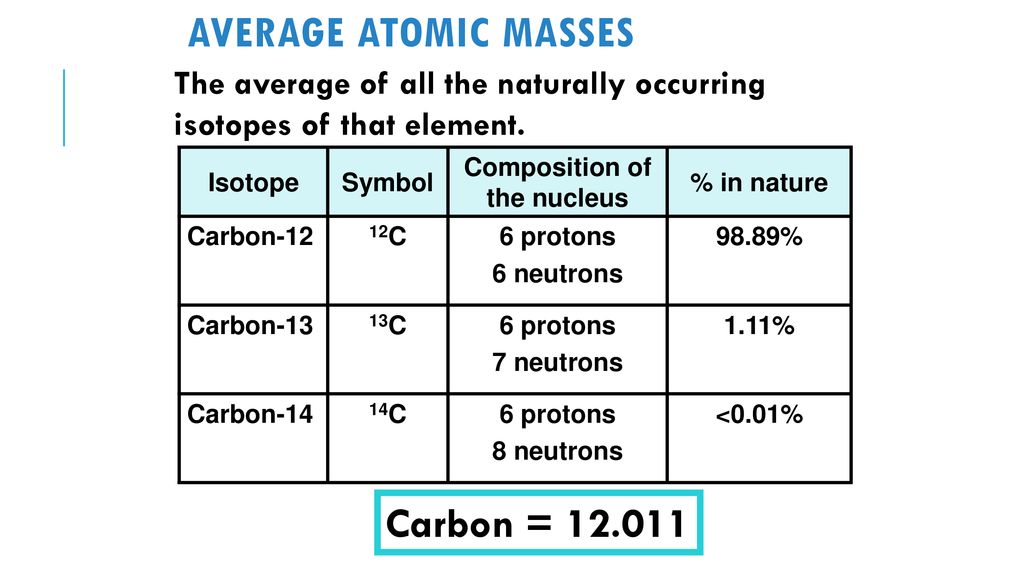
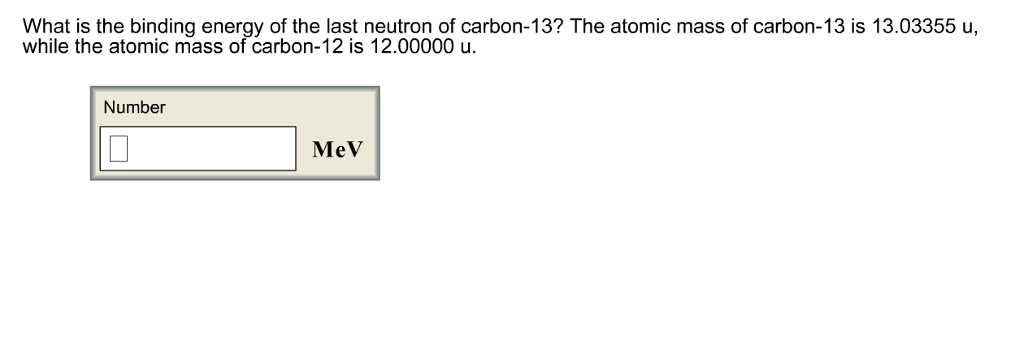
SOLVED:The two most abundant naturally occurring isotopes of carbon are carbon- 12(98.90 %, 12.000 amu) and carbon-13 (1.10 %, 13.003 amu). From these abundances, calculate the atomic weight of carbon and compare

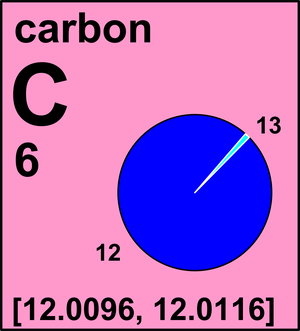
Carbon occurs in the nature as a mixture of carbon-12 and carbon-13 The average atomic mass of carbon is 12 - Science - Structure of the Atom - 13448119 | Meritnation.com
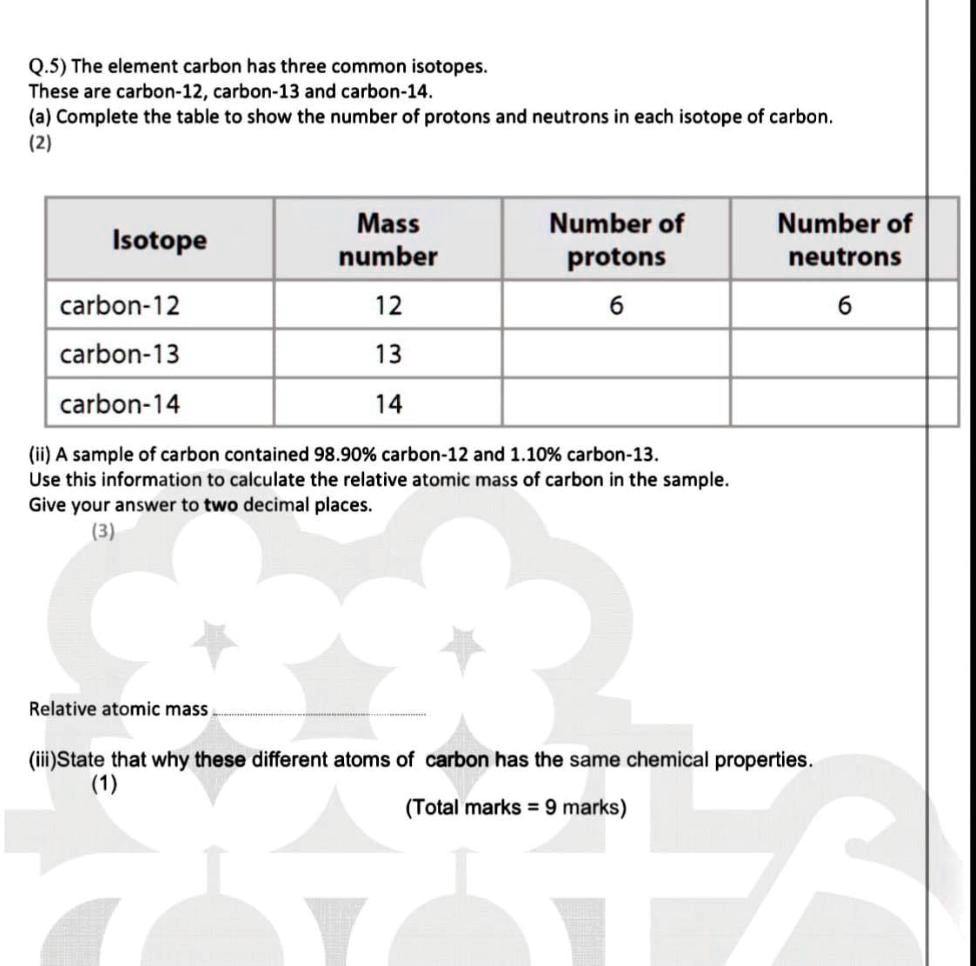
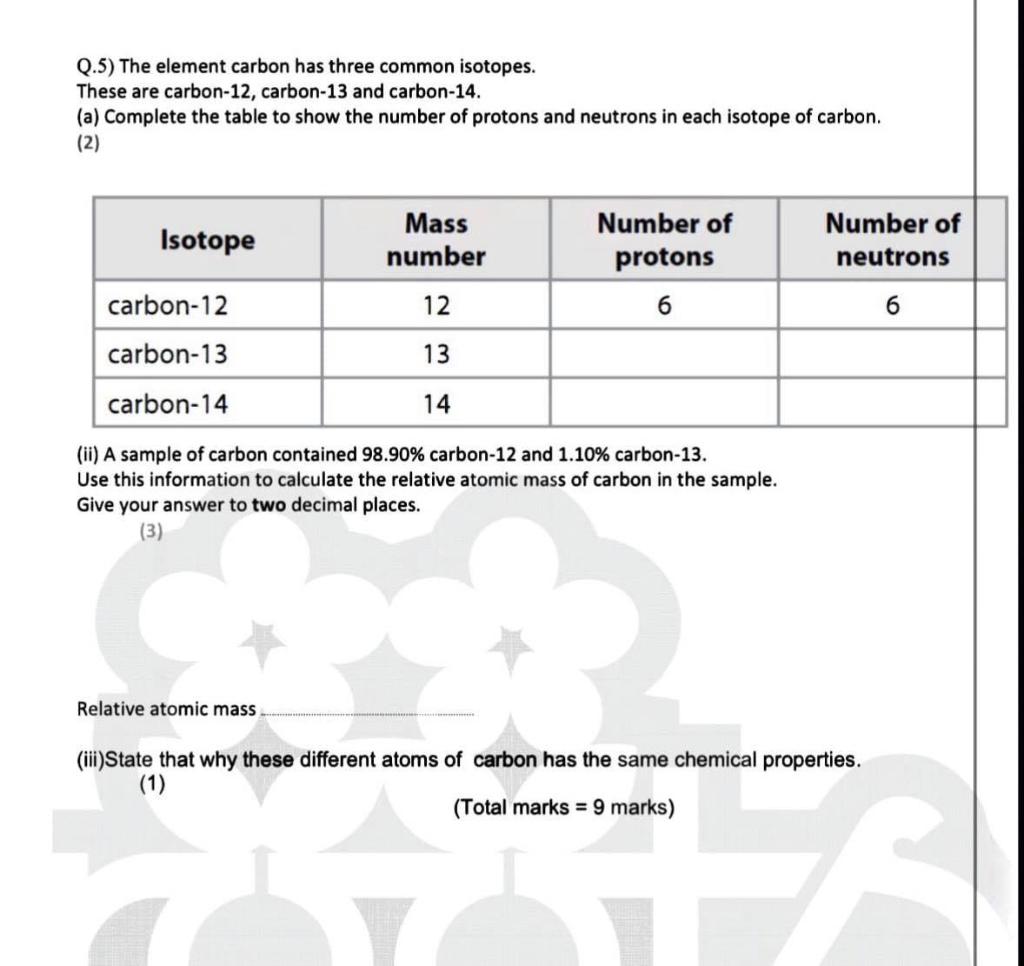
SOLVED: Q.5) The element carbon has three common isotopes: These are carbon-12, carbon-13 and carbon-14 (a) Complete the table to show the number of protons and neutrons in each isotope of carbon: (

SOLVED: Calculate the average atomic mass of carbon if 98.90% of the atoms are C-12 (12.000000 amu) and 1.100% are C-13 atoms (13.003354 amu).

Atomic Mass Standard mass unit is derived from carbon 12 Atomic mass unit – the mass equal to 1/12 the mass of one Carbon 12 atom. - ppt download

Carbon occur in nature as a mixture of C-12 and C-13. Average atomic mass of carbon is 12.011 what is the % abundance of C-12 in nature ?
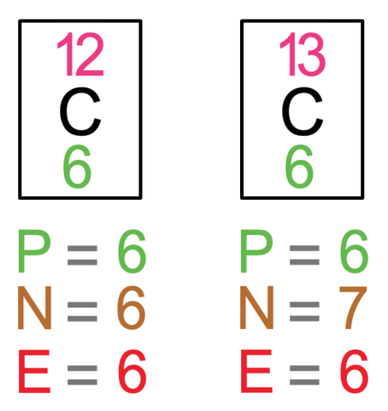
SOLVED:List the mass number and atomic number of carbon-12 and carbon-13, respectively. a. The mass number and atomic number of carbon-13 is 13 and 6, while that of carbon-12 is 12 and

The common isotopes of carbon are ^12C and ^13C . The average mass of carbon is 12.01115 amu. What is the abundance of ^13C isotope ?.