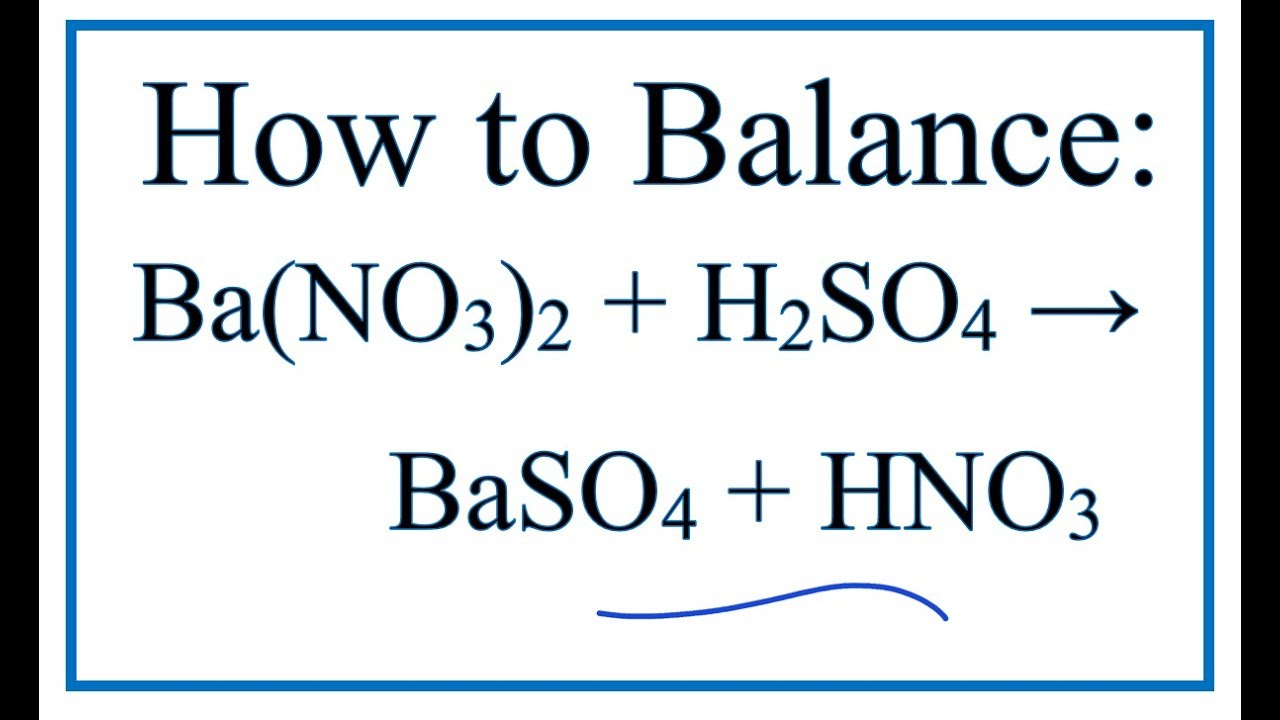
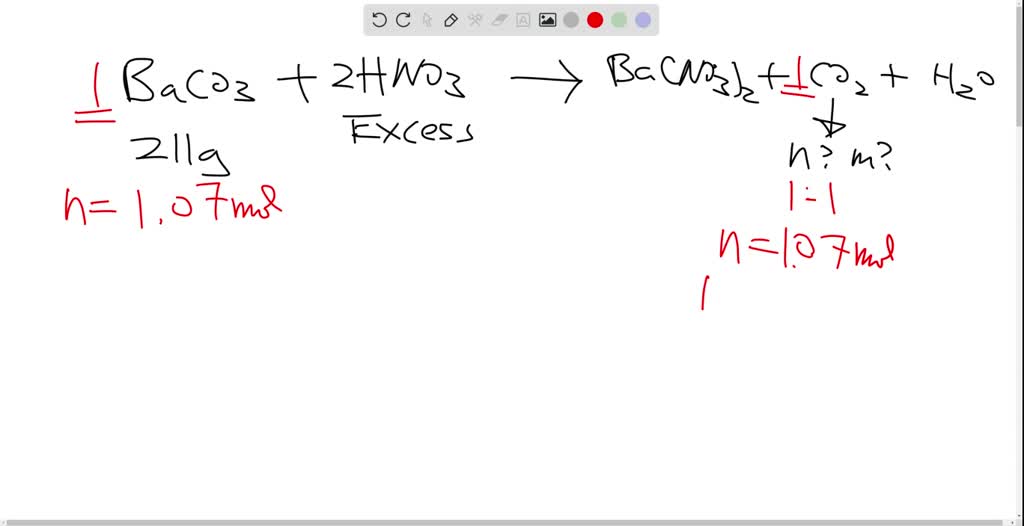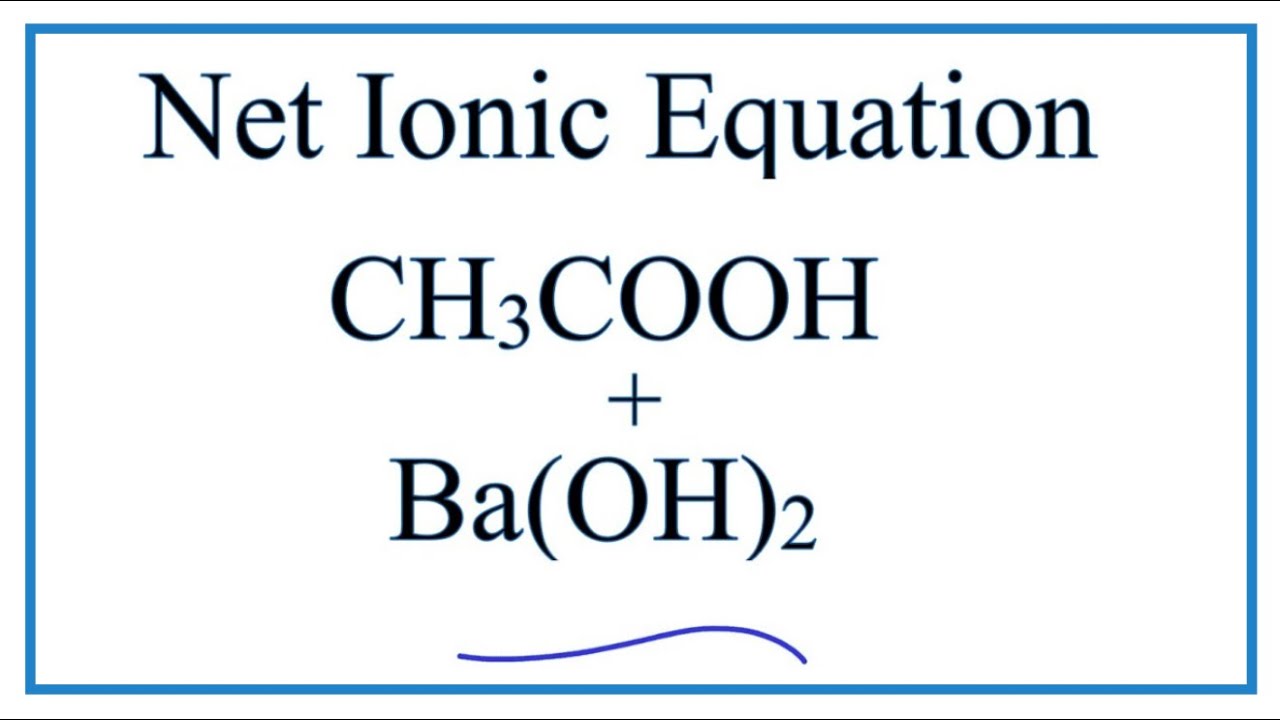SOLVED:A 211 g sample of barium carbonate, BaCO 3, reacts with a solution of nitric acid to give barium nitrate, carbon dioxide and water. If the acid is present in excess, what

Scharlab Barium, standard solution 1000 mg/l Ba for ICP(barium carbonate in nitric acid 2%), 100 mL, MA: Products | Fisher Scientific
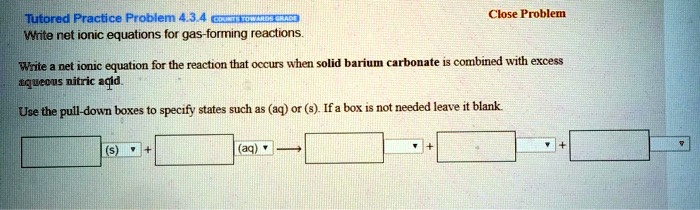
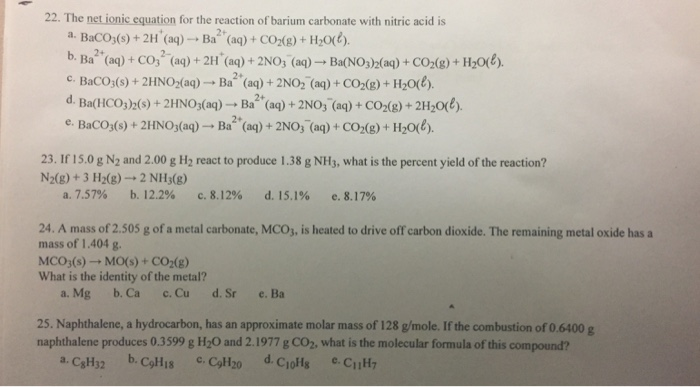
SOLVED: Tutorad Practice Prablem 43 Wtite net ionic equations lor gas-forming reactions Close Probler Wrte net ionc equation for the reaction that occurs when solid barium carbonate combined with excess tquecus nitric

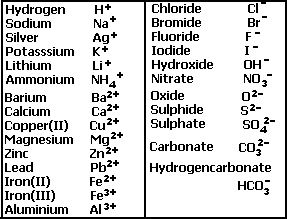
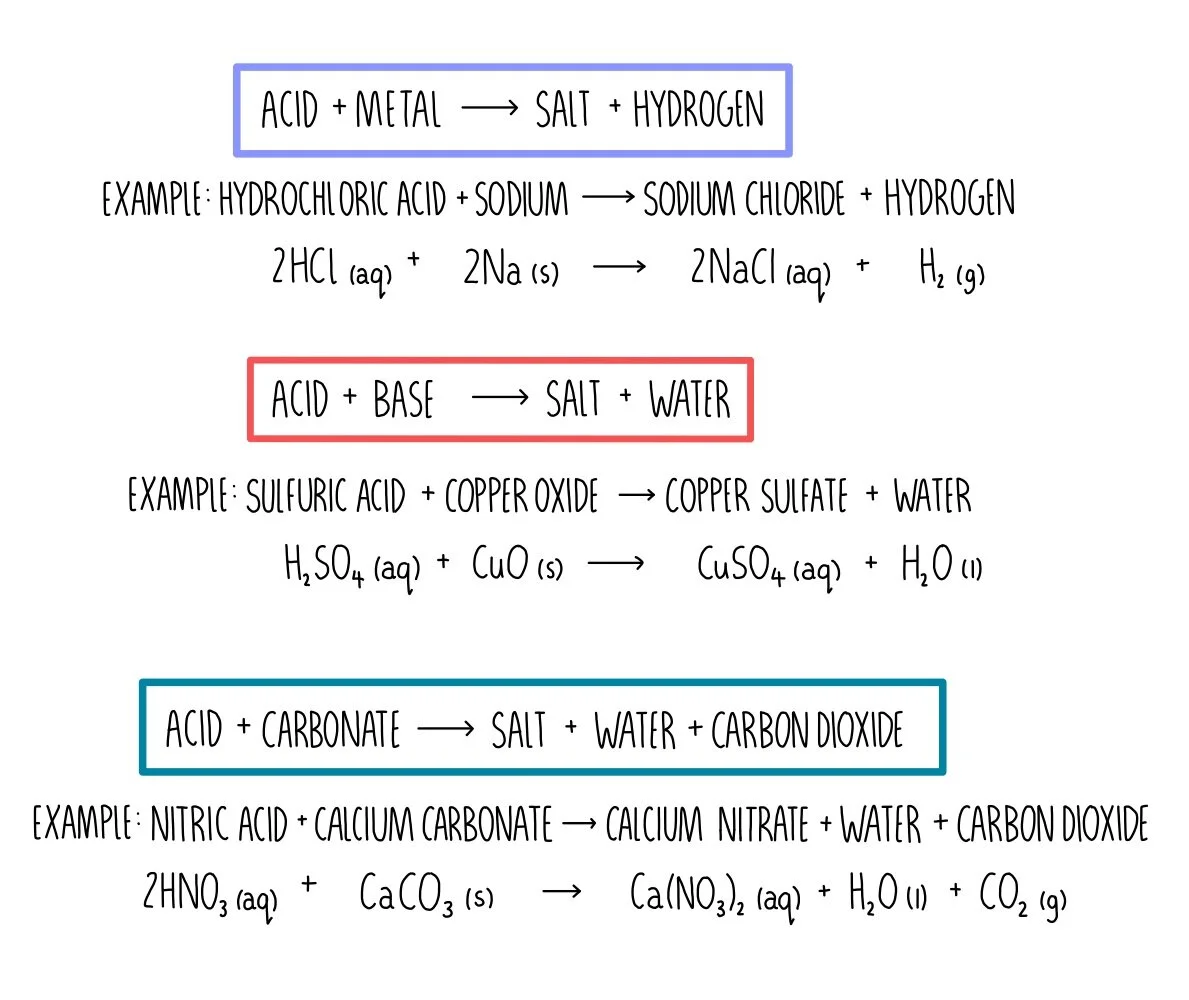
Write the balanced chemical equations for the following reactions.(a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water(b) Zinc + Silver nitrate → Zinc nitrate + Silver(c) Aluminium + Copper chloride

SciELO - Brasil - Determination and fractionation of barium in Brazil nuts Determination and fractionation of barium in Brazil nuts

OneClass: Write a net ionic equation for the reaction that occurs when excess nitric acid (aq) and so...

Starting with barium carbonate solid, dilute sulphuric acid and dilute nitric acid,describe how you would prepare dry barium sulphate solid