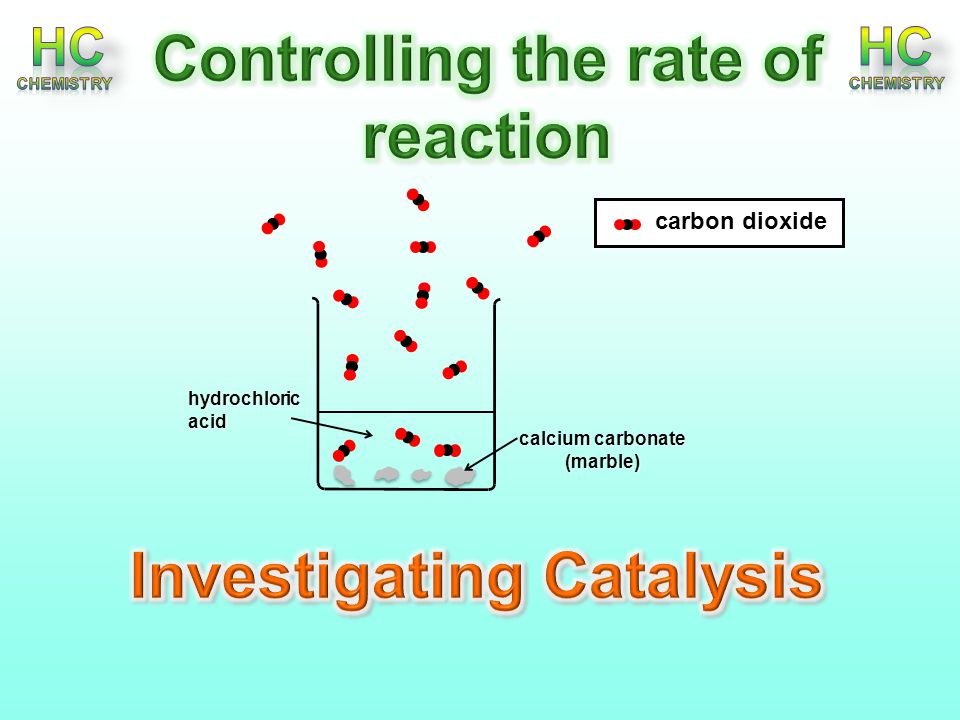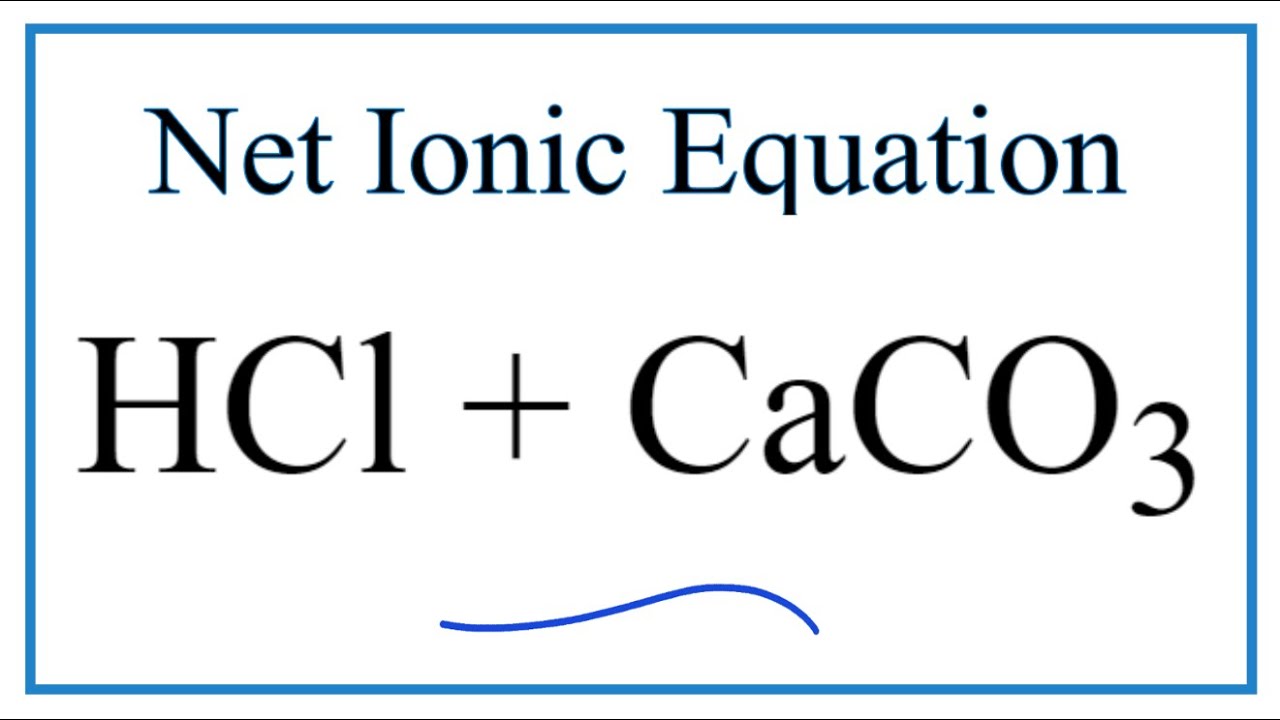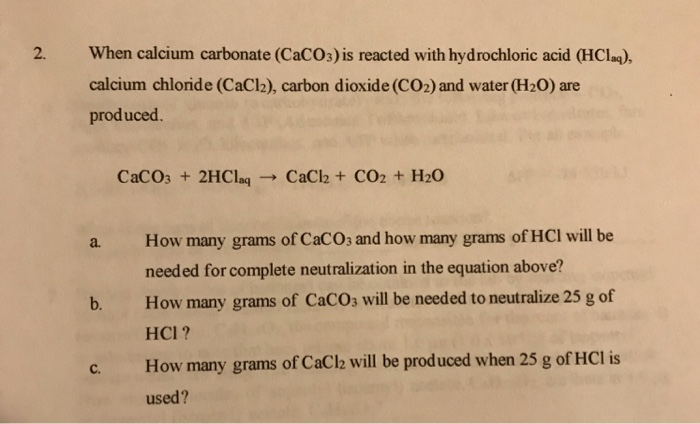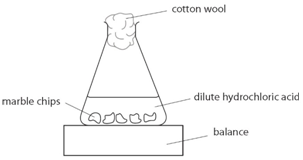
3:15 practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric

Question Video: Calculating the Average Rate of Reaction of Hydrochloric Acid with Calcium Carbonate | Nagwa

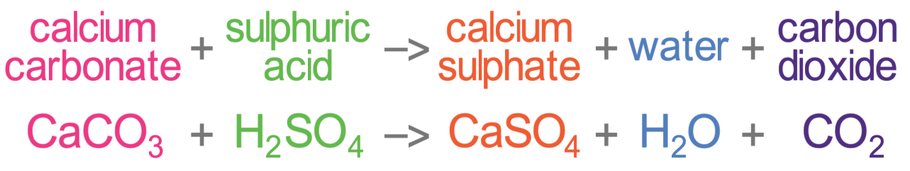
write the chemical equation when calcium carbonate reacts with dilute hydrochloric acid and - Brainly.in

SOLVED: The active ingredients in a tablet of a popular antacid are listed as 585 mg of CaCO3 and 112 mg of Mg(OH): These each react with hydrochloric acid found in your

CaCO3 + 2HCl → CaCl2 + H2O + CO2 The mass of calcium chloride formed when 2.5 g of calcium carbonate is dissolved in excess of hydrochloric acid is:

Chemical reactions of two calcium salts (citrate and carbonate) with... | Download Scientific Diagram

Question Video: Writing a Net Ionic Equation for the Reaction of Solid Calcium Carbonate with a Hydrochloric Acid Solution | Nagwa