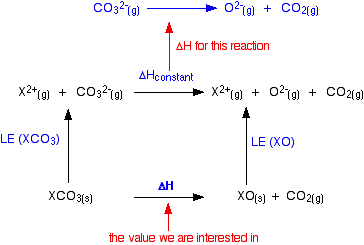
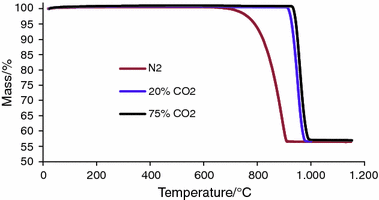
Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect
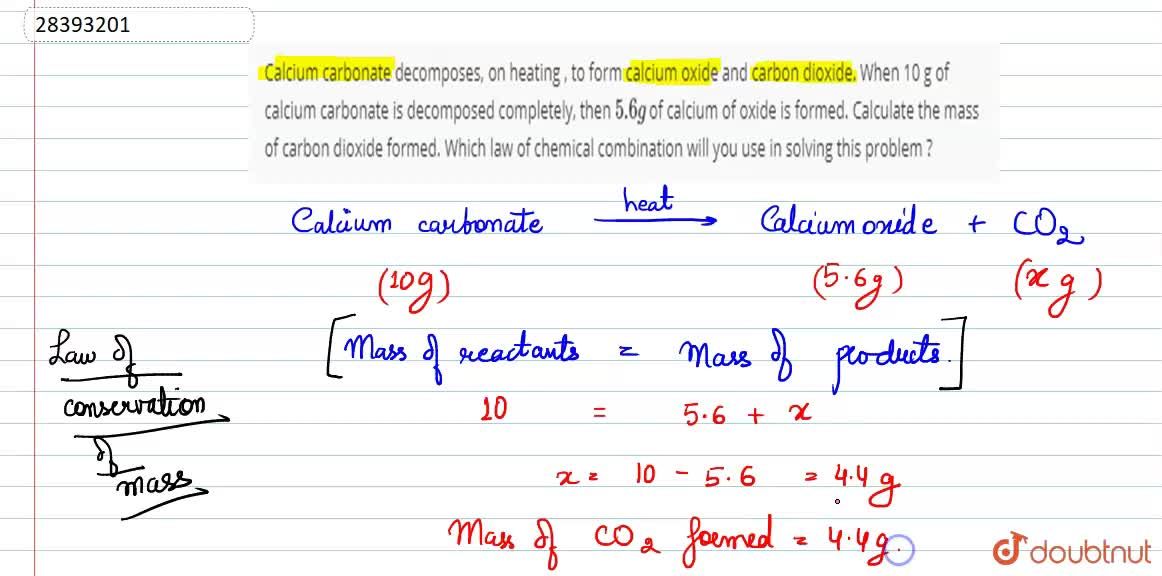
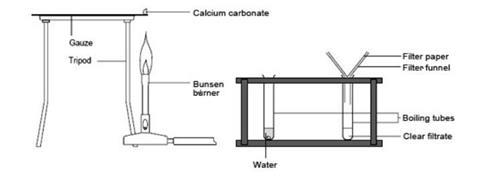
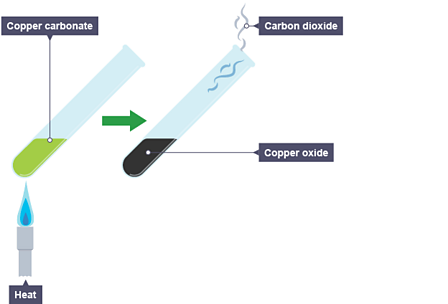
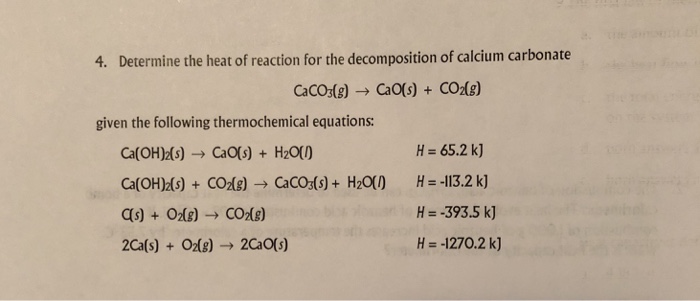
Calcium carbonate decomposes, on heating , to form calcium oxide and carbon dioxide. When 10 g of calcium carbonate is decomposed completely, then 5.6 g of calcium of oxide is formed. Calculate

Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction - ScienceDirect

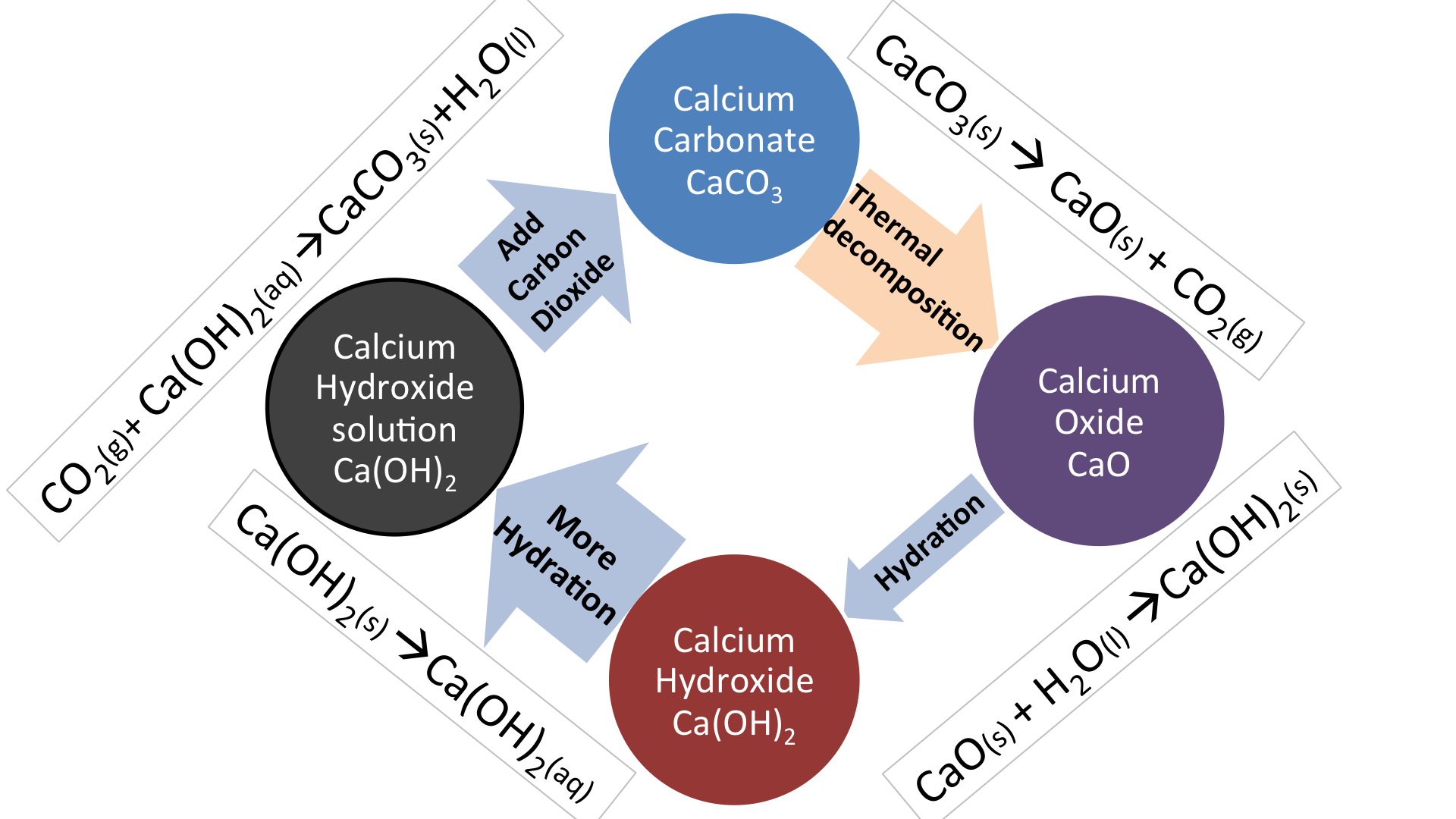
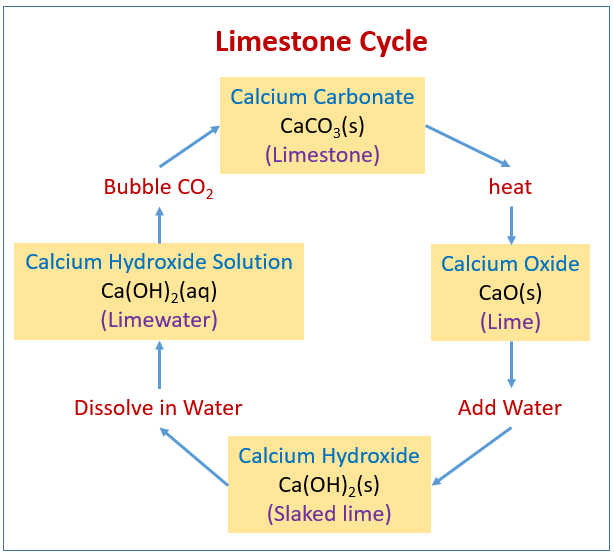
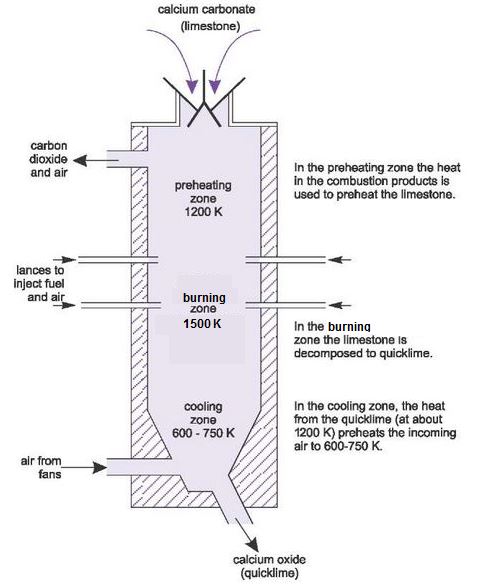
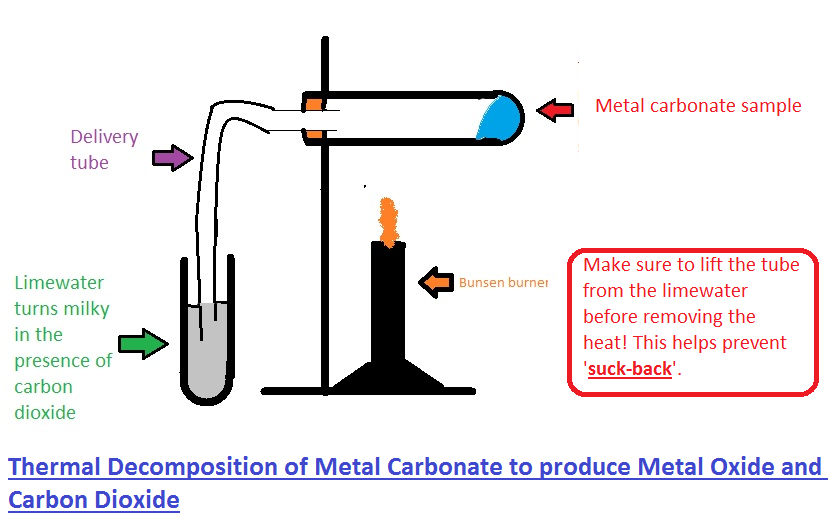
Uses Limestone cycle manufacture cement concrete glass clay ceramics thermal decomposition carbonates, nitrates, slaked lime calcium oxide hydroxide quarrying issues gcse igcse O level chemistry revision notes