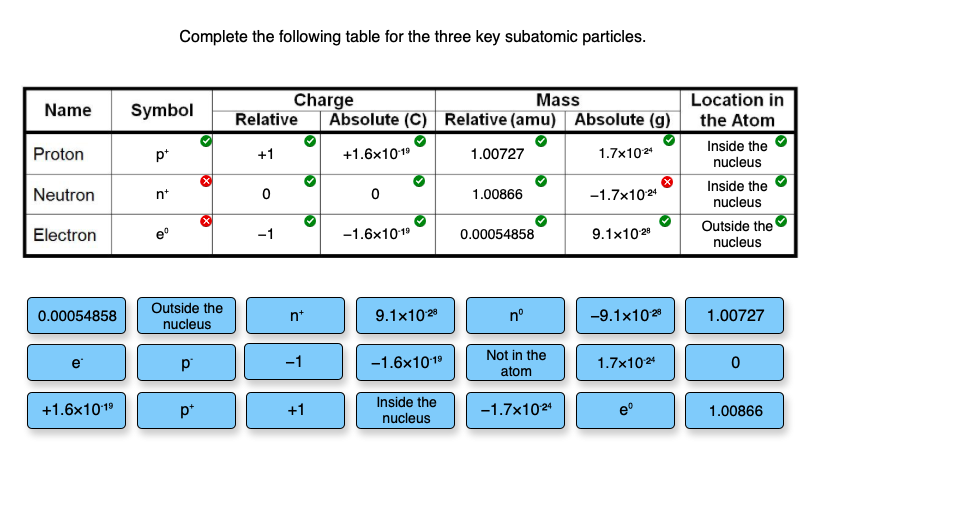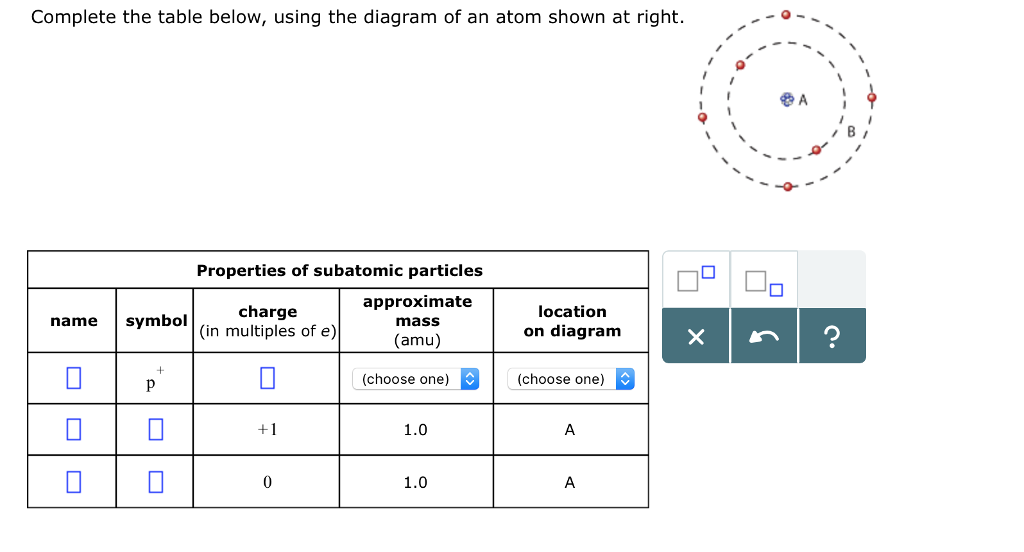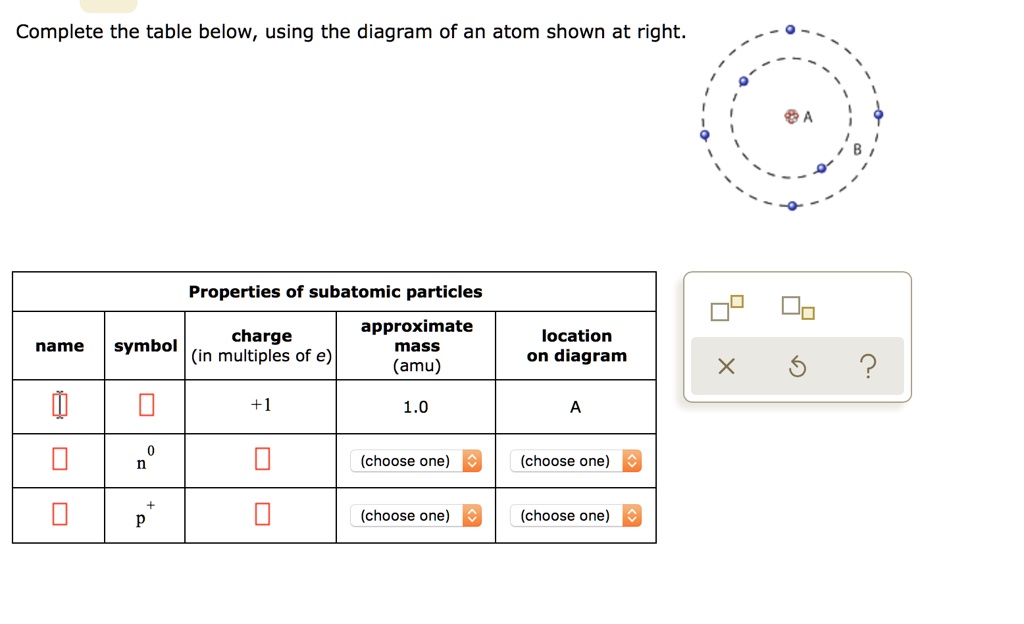
SOLVED: Complete the table below, using the diagram of an atom shown at right: Properties of subatomic particles approximate symbol charge name mass (in multiples of e) (amu) location on diagram 2 +

Subatomic Particles. 1. Subatomic Particles ParticleSymbol (table O) ChargeMass (amu) Location Electrons e 0 e -1 0 β -1 Negative (-1) 1/1872 amu 0 amu. - ppt download
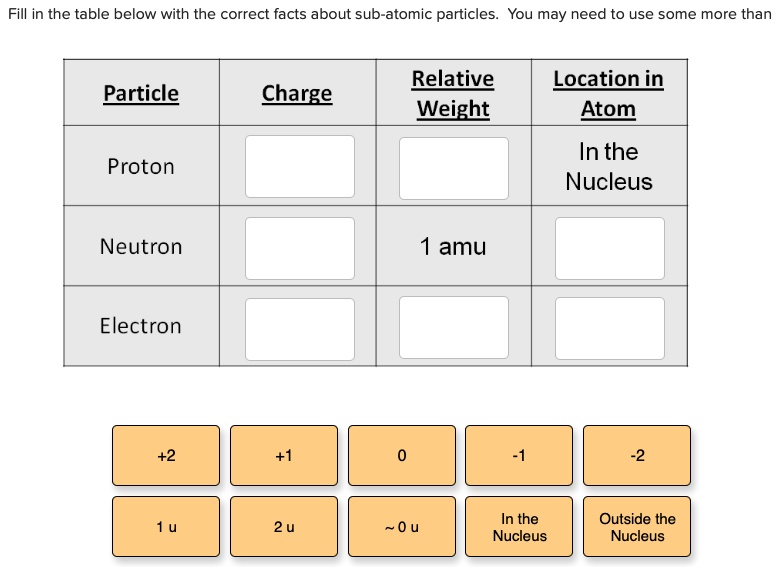
SOLVED: Fill in the table below with the correct facts bout sub-atomic particles: You may need to use some more than Relative Weight Locationin Atom Particle Charge In the Nucleus Proton Neutron

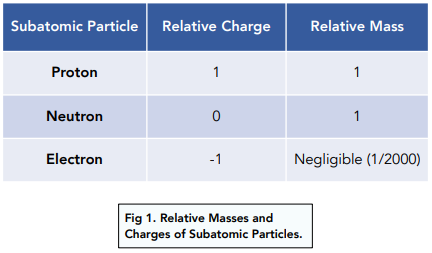
Particles in the Atom & Atomic Structure (1.2.1) | CIE A Level Chemistry Revision Notes 2019 | Save My Exams

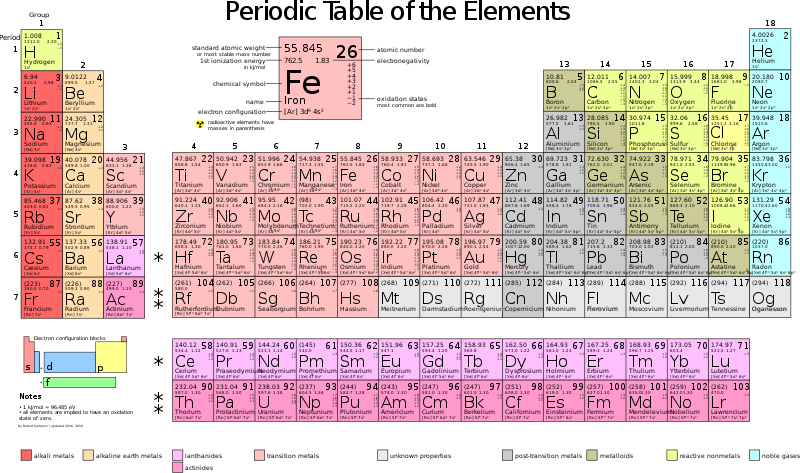
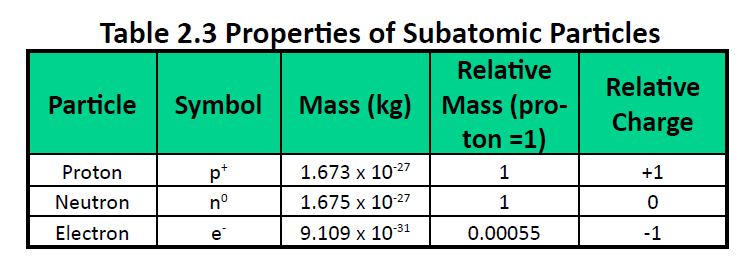
Atomic Structure & the Periodic Table (3.1.1) | CIE IGCSE Chemistry Revision Notes 2022 | Save My Exams