
Sulfoxaflor Insecticide Molecule. Stylized Skeletal Formula Chemical Structure: Atoms are Shown As Color-coded Circles. Stock Illustration - Illustration of pesticide, atomic: 187938441

Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species | Scientific Reports
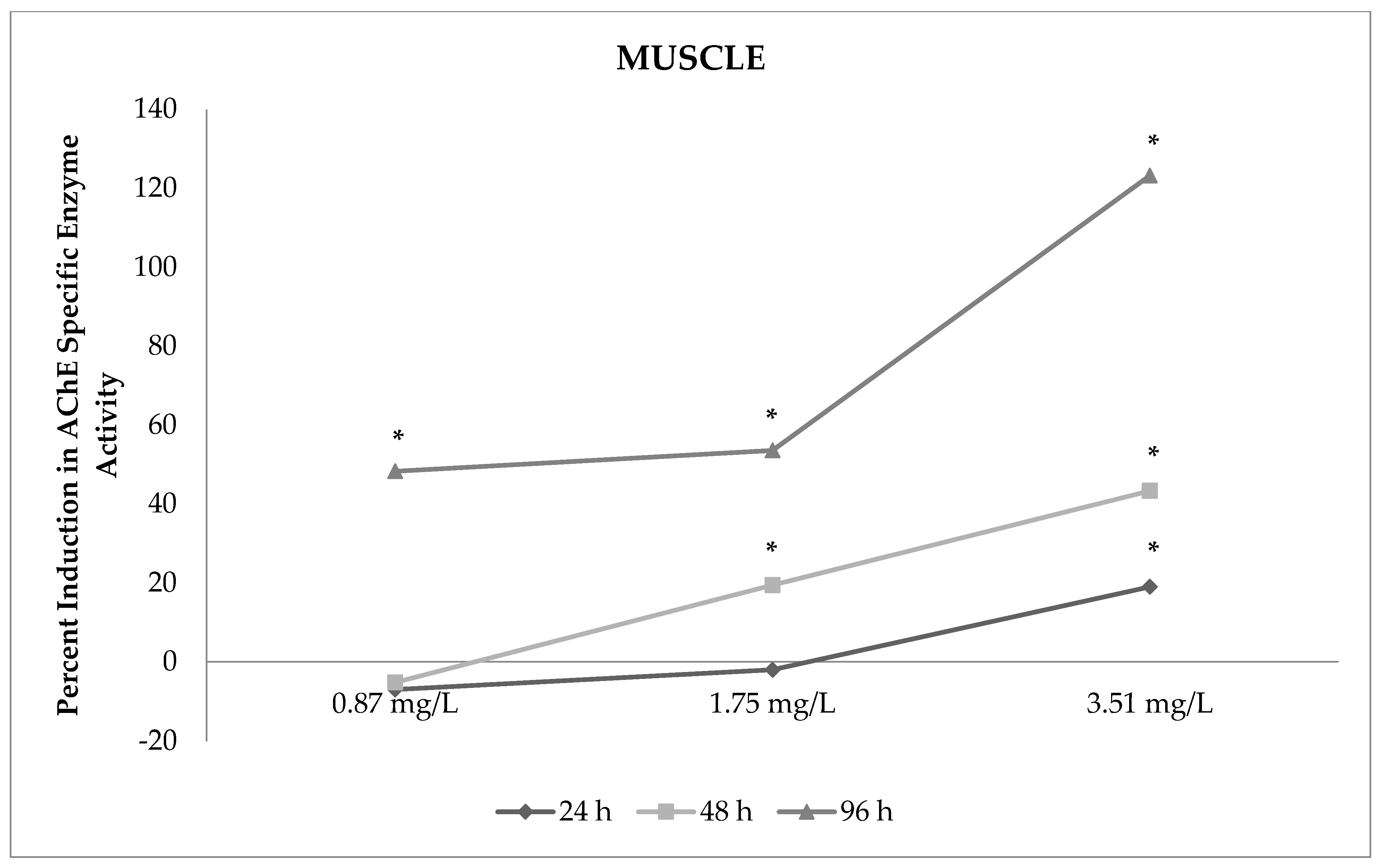
Toxics | Free Full-Text | In Vivo Effects of Neonicotinoid-Sulfoximine Insecticide Sulfoxaflor on Acetylcholinesterase Activity in the Tissues of Zebrafish (Danio rerio)

Molecules | Free Full-Text | Synthesis, Physicochemical Properties, and Biological Activities of 4-(S-Methyl-N-(2,2,2-Trifluoroacetyl)Sulfilimidoyl) Anthranilic Diamide
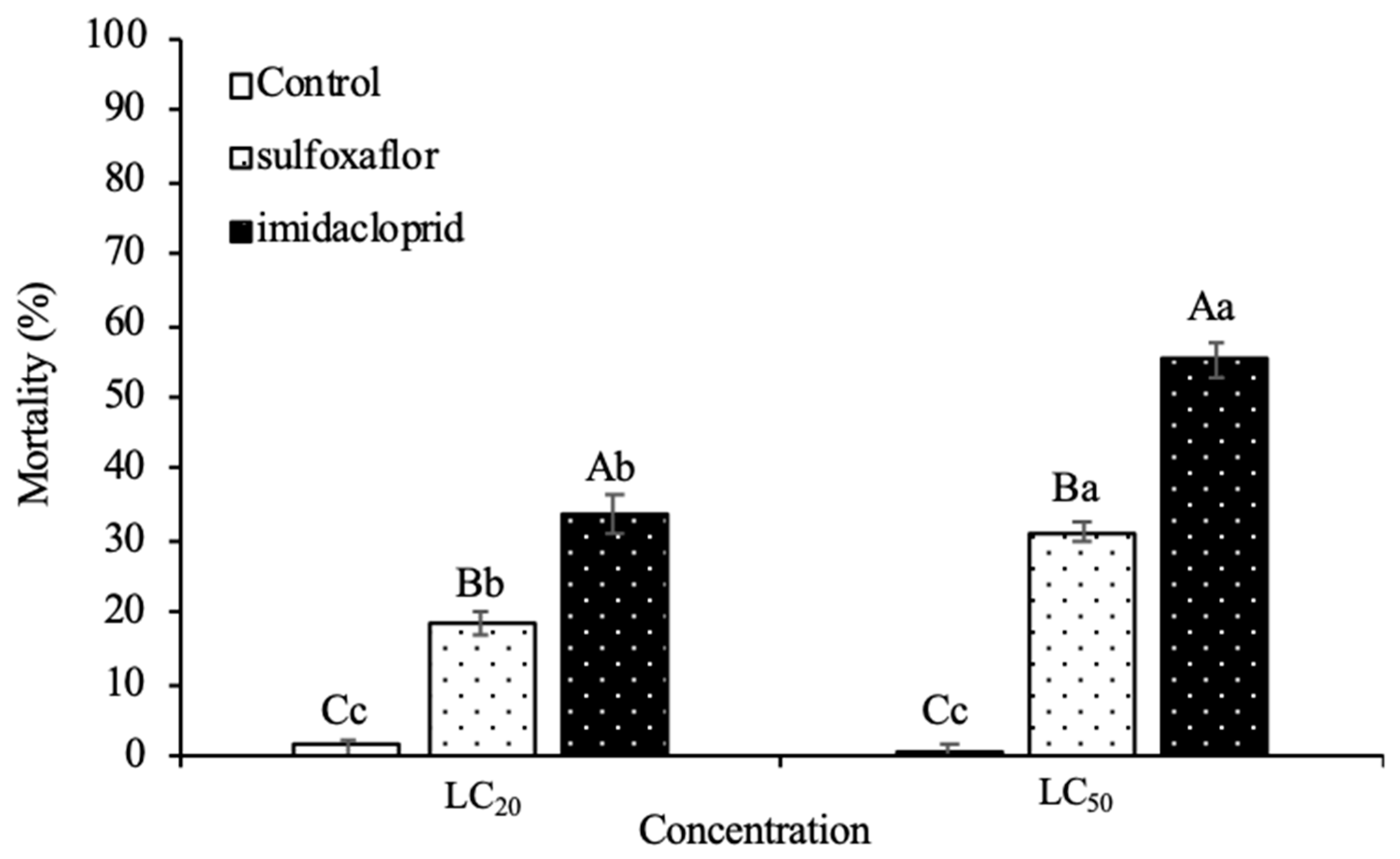
Insects | Free Full-Text | Transgenerational Effects of a Neonicotinoid and a Novel Sulfoximine Insecticide on the Harlequin Ladybird

Sulfoxaflor insecticide molecule. Stylized skeletal formula (chemical structure): Atoms are shown as color-coded circles: hydrogen (hidden), carbon (grey), oxygen (red), nitrogen (blue), sulfur (yellow), fluorine (cyan Stock Photo - Alamy

New chemicals expected to replace banned pesticides also pose significant threat to bees | The Independent | The Independent

Synthesis route for commercial sulfoximine insecticide Sulfoxaflor 174 | Download Scientific Diagram

Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects - ScienceDirect

Evolution of sulfoximine analogs leading to sulfoxaflor, structures of... | Download Scientific Diagram

Differential metabolism of sulfoximine and neonicotinoid insecticides by Drosophila melanogaster monooxygenase CYP6G1 - ScienceDirect

Neonicotinoid and sulfoximine pesticides differentially impair insect escape behavior and motion detection | PNAS

Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects - ScienceDirect

The sulfoximine insecticide sulfoxaflor exposure reduces the survival status and disrupts the intestinal metabolism of the honeybee Apis mellifera - ScienceDirect

Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects - ScienceDirect

Sulfoxaflor – A sulfoximine insecticide: Review and analysis of mode of action, resistance and cross-resistance - ScienceDirect
Lethal and sub-lethal effects of a novel sulfoximine insecticide, sulfoxaflor, against Asian citrus psyllid and its primary para

Synthesis route for commercial sulfoximine insecticide Sulfoxaflor 174 | Download Scientific Diagram





